Sialic acid (Sia) is sometimes linked to each other to form polysialic acid (polySia) chain in prokaryotic and eukaryotic organisms. The α2,8-linked polySia structures occur in capsular polysaccharides of neuroinvasive bacteria. In eukaryotes, α2,8-polySia chain is present on salmonid egg polysialoglycoprotein (PSGP), neural cell adhesion molecule (NCAM), sodium channels, CD36 and so on. polySia is widely distributed in nature from bacteria to human. PolySia-NCAM is abundantly expressed in vertebrate embryonic brain. Two polysialyltransferases, PST (ST8Sia IV) and STX (ST8Sia II), are responsible for the biosynthesis of polySia chain on N-linked glycan of NCAM. Both PST and STX can catalyze the formation of polySia chains on α2,3- or α2,6-sialylated N-linked glycans of NCAM. In contrast to NCAM with polySia on N-glycans, PSGP contains the polySia on O-linked glycans. PSGP is a cortical alveolus glycoprotein of fish oocyte. PSGP consists of tandem repeats of tridecaglycopeptide carrying three O-linked glycans with polySia chain which is an α2,8-linked polySia with chain length of up to 25 Sia residues. No N-glycan is present in PSGP. The polySia chain in PSGP is also synthesized by PST and STX in rainbow trout ovary. Notably, the co-existence of PST and STX in the reaction mixture synergistically enhanced the α2,8-polyST activity. Here describes the assay method for the polysialyltransferase activity in vertebrates. However, the same protocol is basically applicable to that in prokaryotes. |
| Category | Glycosyltransferases & related proteins |
| Protocol Name | Enzyme assay of polysialyltransferase |
Authors
 |
Kitajima, Ken
*
Bioscience and Biotechnology Center, Nagoya University
Sato, Chihiro
Bioscience and Biotechnology Center, Nagoya University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
CMP-[14C]Neu5Ac (12.4 GBq/mmol; GE Healthcare, Little Chalfont, UK) |
| ● |
NCAM-Fc (the soluble human neural cell adhesion molecule fused with Fc region of human IgG1) |
| ● |
pPROTA vector (kindly provided by Dr. John Lau, Roswell Park Cancer Institute, Buffalo, NY) |
| ● |
COS-1 cells (kindly provided by Dr. Karen Colley, University of Illinois at Chicago, IL) |
| ● |
pcDNA hPST-V5 (kindly provided by Dr. Karen Colley) |
| ● |
pcDNA hSTX-V5 (kindly provided by Dr. Karen Colley) |
| ● |
Mouse monoclonal antibody 12E3, which recognizes (Neu5Ac)n (n ≥ 5) (kindly gifted from Dr. Tatsunori Seki, Nihon Medical University School of Medicine) |
| ● |
anti-myc (mouse monoclonal antibody; kindly gifted from Dr. Rita Gerardy-Schahn, Medizinische Hochschule Hannover, Germany) |
| ● |
anti-V5 (mouse monoclonal antibody; Invitrogen/Life Technologies, Carlsbad, CA) |
|
Instruments
 |
| ● |
Whatman 3MM paper (approximately 15 cm × 10 cm) |
| ● |
A tank for paper chromatography (glassware; Depth 15 cm × Width 25 cm × Height 25 cm) |
| ● |
BAS 2000 imaging analyzer (Fujifilm, Tokyo, Japan) |
|
| Methods |
|
1. |
Enzyme assay of polysialyltransferase |
| 3) |
Prepare the reaction mixture (10 μL) containing 4 μL of the recombinant enzyme, 50 mM MES buffer (pH 6.0), 10 mM MnCl2, 10 mM CaCl2, 0.5% Triton CF-54, 100 mM CMP-[14C]Neu5Ac (10.7 kBq), and the acceptor substrate. |
Comment 0
|

|
| 4) |
Incubate at 25℃ or 37℃ for 24 h. |
Comment 1
|

|
| 5) |
Spot a 5-μL aliquot of reaction mixture on Whatman 3MM paper, immediately followed by spotting of 15 μL of ethanol to terminate the reactions. |
Comment 0
|

|
| 6) |
Develop with ethanol/1 M ammonium acetate (pH 7.5) (7:3, v/v) for 30 min. |
Comment 0
|

|
| 7) |
After air-drying, the amount of incorporated [14C]Neu5Ac remaining at the origin was determined by a BAS 2000 imaging analyzer. |
Comment 0
|

|
| 8) |
Determine the amount of enzyme used. The amount of fusion protein adsorbed to IgG-Sepharose was analyzed by Western blotting using the antibody against the epitope-tag. |
Comment 0
|
|
|
| Figure & Legends |
Figure & Legends 
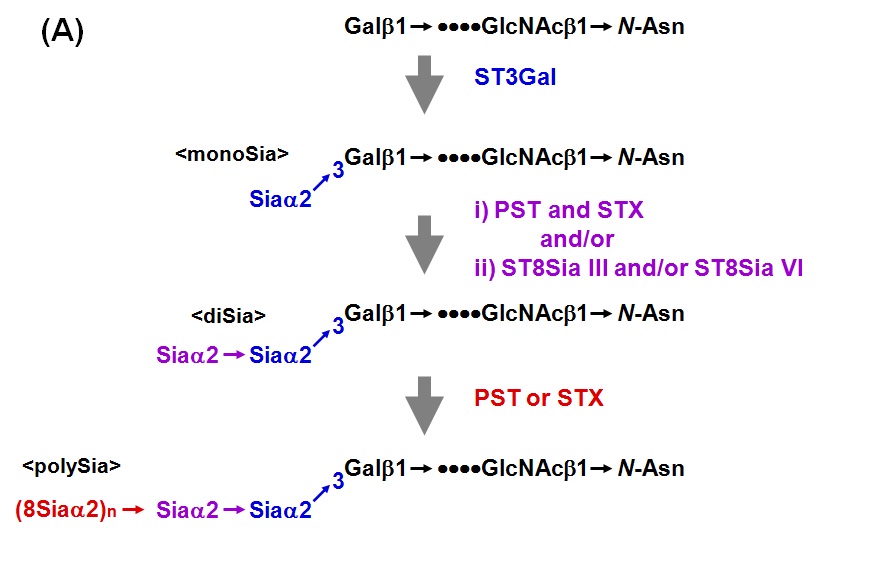
Fig. 1A. Biosynthesis of polySia in N-linked glycan chains of the neural cell adhesion molecule (NCAM)

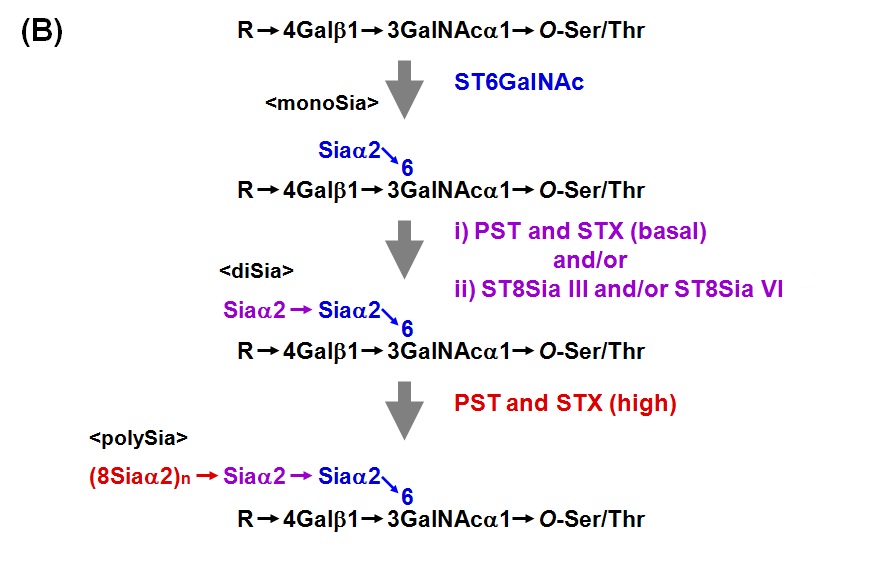
Fig. 1B. Biosynthesis of polySia in O-linked glycan chains of fish egg polysialoglycoprotein (PSGP) |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-29 16:19:09 |
- Asahina, S., Sato, C., Matsuno, M., Matsuda, T., Colley, K., and Kitajima, K. (2006) Involvement of the α2,8-polysialyltransferases II/STX and IV/PST in the biosynthesis of polysialic acid chains on the O-linked glycoproteins in rainbow trout ovary. J Biochem. 140, 687–70 [PMID : 17023684]
- Asahina, S., Sato, C., and Kitajima, K. (2004) Developmental expression of a sialyltransferase responsible for sialylation of cortical alveolus glycoprotein during oogenesis in rainbow trout (Oncorhynchus mykiss). J Biochem. 136, 189–198 [PMID : 15496590]
- Sato, C., Kitajima, K., Tazawa, I., Inoue, Y., Inoue, S., and Troy, F.A. (1993) Structural diversity in the α2→8-linked polysialic acid chains in salmonid fish egg glycoproteins. Occurrence of poly(Neu5Ac), poly(Neu5Gc), poly(Neu5Ac, Neu5Gc), poly(KDN), and their partially acetylated forms. J. Biol. Chem. 268, 23675–23684 [PMID : 8226894]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Kitajima, Ken,
Sato, Chihiro,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.22,10,2024 .
How to Cite this Work in Website:
Kitajima, Ken,
Sato, Chihiro,
(2014).
Enzyme assay of polysialyltransferase.
Retrieved 22,10,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t67.
html source
Kitajima, Ken,
Sato, Chihiro,
(2014).
<b>Enzyme assay of polysialyltransferase</b>.
Retrieved 10 22,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t67" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t67</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|