Fucosylation is one of the most important oligosaccharide modifications involved in cancer and inflammation. Fucosylation is divided into several types, including α 1-2, α 1-3, α 1-4, and α 1-6 (core fucose). Alpha-fetoprotein (AFP) can be glycosylated into several isoforms, and it is specifically the α 1-6 fucosylated isoform (AFP-L3) that serves as a key biomarker for hepatocellular carcinoma. This oligosaccharide structure can be detected by lectin affinity electrophoresis using Lens culinaris agglutinin (LCA). LCA can also be used for affinity chromatography, but is not available for lectin blot analysis. Conventionally, in addition to LCA, other commercially available core fucose-binding lectins, such as Pisum sativum agglutinin, Aleuria aurantia lectin (AAL) and Aspergillus oryzae lectin have been used in studies on glycobiology. However, these lectins do not bind solely to core fucose. Pholiota squarrosa lectin (PhoSL) is a novel lectin recently purified from mushroom. PhoSL can bind α 1-6 fucosylation more specifically, as shown in the content of PhoSL lectin by Yuka Kobayashi. Fucosylation and loss of fucosylation are directly involved in cancer progression and, especially, core fucosylation plays pivotal roles in cellular signaling via oligosaccharide modification of cell surface growth factor receptors 1). In this protocol, we describe the methods for PhoSL immunostaining for the purpose of better understanding the biological significance of core fucosylation in cancer. |
| Category | Sugar binding proteins |
| Protocol Name | Core fucose-specific lectin Pholiota squarrosa lectin and its application to histochemistry |
Authors
 |
Nakayama, Kotarosumitomo
Department of Molecular Biochemistry and Clinical Investigation, Osaka University Graduate School of Medicine
Miyoshi, Eiji
*
Department of Molecular Biochemistry and Clinical Investigation, Osaka University Graduate School of Medicine
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
|
| ● |
70, 80, 90, and 100% ethanol |
| ● |
Phosphate-buffered saline (PBS) |
| ● |
Tris-buffered saline containing 0.05% Tween 20 (TBST) |
| ● |
Biotin blocking solution: Biotin-Blocking System (Dako, Glostrup, Denmark, #X0590) |
| ● |
Avidin blocking solution: Biotin-Blocking System (Dako, #X0590) |
| ● |
Endogenous peroxidase blocking solution: Peroxidase-Blocking Solution, Dako REALTM (Dako, #S2023) |
| ● |
Blocking solution: 5% bovine serum albumin (BSA) in TBST |
| ● |
PhoSL-biotin: diluted by 20 μg/mL with blocking solution (5% BSA in TBST; ready to use) |
| ● |
Horseradish peroxidase (HRP)-conjugated avidin biotin complex (ABC): VECTASTAIN ABC Standard Kit (#PK-4000, Vector Laboratories, Inc., Burlingame, CA) diluted by 1/50 with PBS (ready to use) |
| ● |
DAB chromogenic substrate: 1/50 DAB+, Liquid (Dako, #K3467) (ready to use) |
|
Instruments
 |
| ● |
|
| ● |
MAS coated slide (Matsunami Glass Ind., Ltd., Osaka, Japan, #S9941) |
| ● |
|
| ● |
|
| ● |
|
| ● |
Cancerous and normal tissues from human (Tissue array, KURABO Industries Ltd., Osaka, Japan) |
|
| Methods |
|
1. |
Preparation of tissue section for staining |
| 1) |
Tissues are preserved with 10% formalin in PBS and embedded in paraffin. |
Comment 0
|

|
| 2) |
Cut sections 3 to 5μm thick, and mount the sections on MAS coated slides. |
Comment 1
|

|
| 3) |
Immerse the slides in fresh xylene three times for 5 min each. |
Comment 0
|

|
| 4) |
Immerse the slides in 100% ethanol for 1 min. |
Comment 0
|

|
| 5) |
Immerse the slides in 90% ethanol for 1 min. |
Comment 0
|

|
| 6) |
Immerse the slides in 80% ethanol for 1 min. |
Comment 0
|

|
| 7) |
Immerse the slides in 70% ethanol for 1 min. |
Comment 0
|

|
| 8) |
Wash the slides with PBS three times for 5 min each. |
Comment 0
|
|
|
|
2. |
Blocking nonspecific binding |
| 1) |
Add biotin blocking solution to the slides according to the manufacturer’s protocols. |
Comment 0
|

|
| 2) |
Incubate the slides at room temperature for 10 min in moist conditions. |
Comment 0
|

|
| 3) |
Wash the slides with PBS two times for 5 min each. |
Comment 0
|

|
| 4) |
Add avidin blocking solution to the slides according to the manufacturer’s protocols. |
Comment 0
|

|
| 5) |
Incubate the slides at room temperature for 10 min in moist conditions. |
Comment 0
|

|
| 6) |
Wash the slides with PBS two times for 5 min each. |
Comment 0
|

|
| 7) |
Add endogenous peroxidase blocking solution to the slides. |
Comment 0
|

|
| 8) |
Incubate the slides at room temperature for 10 min in moist conditions. |
Comment 0
|

|
| 9) |
Wash the slides with PBS three times for 5 min each. |
Comment 0
|

|
| 10) |
Immerse the slides in blocking solution (5% BSA in TBST) overnight at 4°C. |
Comment 0
|
|
|
|
3. |
Lectin binding and chromogenic reaction |
| 2) |
Incubate the slides at room temperature for 1 h in moist conditions. |
Comment 0
|

|
| 3) |
Wash the slides with PBS for 5 min. |
Comment 0
|

|
| 4) |
Wash the slides with TBST two times for 5 min each. |
Comment 0
|

|
| 5) |
Wash the slides with PBS for 5 min. |
Comment 0
|

|
| 6) |
Add HRP-conjugated ABC to the slides. |
Comment 1
|

|
| 7) |
Incubate the slides at room temperature for 30 min in moist conditions. |
Comment 0
|

|
| 8) |
Wash the slides with PBS three times for 5 min each. |
Comment 0
|

|
| 9) |
Add DAB chromogenic substrate to the slides. |
Comment 0
|

|
| 10) |
Incubate the slides for approximately 1 min under a microscope. |
Comment 1
|

|
| 11) |
Immerse the slides in PBS to stop reaction. |
Comment 0
|

|
| 12) |
View the specimen under the microscope. |
Comment 1
|
|
|
| Notes | Take care to keep slides wet over all the steps; otherwise, the quality of staining will decay. Remove excess fluid surrounding the tissue when you add reagent. |
| Discussion | Many studies have revealed that fucosylation is closely associated with cancer biology through modulation of signal transduction and the cell-cell adhesion pathways1). In general, fucosylation is increased in the early stage of carcinogenesis and fucosylation is thought to be involved in the development of cancer-initiating cells. Fucosylated glycoproteins such as alpha-fetoprotein and haptoglobin can be directly applied to the clinical diagnosis of cancer, especially hepatocellular carcinoma 2). Core and Lewis type fucosylations are increased in cancer, however, possible roles of each fucosylation seem to be different in each type of cancer.
In addition to the cell-cell adhesion pathway, we recently provided new evidence that fucosylation affects tumor immune surveillance via another signaling pathway, the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling pathway 3). Interestingly, fucosylation of TRAIL receptors is not involved in this biological effect. Complex II formation, which is an intra-cellular event in apoptotic cells, is altered with or without fucosylation 4). Since loss of fucosylation is dependent on the inhibition of GDP-fucose synthesis, both core and Lewis type fucosylations are limited in apoptotic cells 3). PhoSL 5) is a possible tool to address whether core or Lewis type fucosylation is involved in TRAIL signaling. It would be interesting to determine whether or not loss of PhoSL staining is associated with escape from TRAIL-induced apoptosis and progression of cancer (see Fig. 2).
Furthermore, a second generation of fucosylated haptoglobin enzyme-linked immunosorbent assay (ELISA) kit could be established using PhoSL instead of AAL. Previous work shows that site-specific analyses of fucosylated haptoglobin gave a unique oligosaccharide structure of site 3 on haptoglobin 6). Therefore, a combination assay of AAL and PhoSL ELISA kits would be a promising tool for cancer diagnosis. Taken together, PhoSL is the most suitable lectin for recognizing core fucose and might play a pivotal role in the future of fucosylation-biology. |
| Figure & Legends |
Figure & Legends

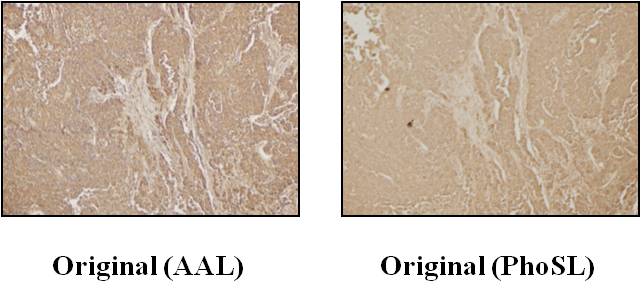
Fig. 1. Immunohistochemical staining of PhoSL in the original cancer tissue.
Left panel: positive staining of AAL in the original colorectal cancer tissues. Most cancer cells showed positive staining for AAL. Staining methods for AAL were similar to those for PhoSL. Right panel: staining for PhoSL, which was consistent with AAL staining.

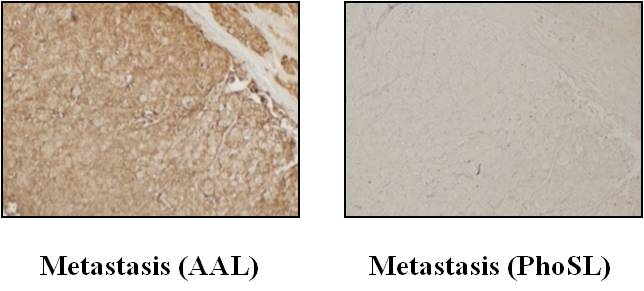
Fig. 2. Immunohistochemical staining of PhoSL in the metastatic lymph nodes of colorectal cancer.
While metastatic cancer cells were stained with AAL, there was no signal for PhoSL staining, suggesting that metastatic cancer cells lack core fucose. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-31 09:29:23 |
- Miyoshi, E., Moriwaki, K., and Nakagawa, T. (2008) Biological Function of Fucosylation in Cancer Biology. J. Biochem. 143, 725–729 [PMID : 18218651]
- Masuda, T., and Miyoshi, E. (2011) Cancer biomarkers for hepatocellular carcinomas: from traditional markers to recent topics. Clin Chem Lab Med. 49, 959–66 [PMID : 21428856]
- Moriwaki, K., Noda, K., Furukawa, Y., Oshima, K., Uchiyama, A., Nakagawa, T., Taniguchi, N., Daigo, Y., Nakamura, Y., Hayashi, N., and Miyoshi, E. (2009) Deficiency of GMD leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology 137, 188–198 [PMID : 19361506]
- Moriwaki, K., Shinzaki, S., and Miyoshi, E. (2011) GMDS deficiency renders colon cancer cells resistant to TRAIL receptor- and CD95-mediated apoptosis by inhibiting complex II formation. J. Biol. Chem. 286, 43123–43133 [PMID : 22027835]
- Kobayashi, Y., Tateno, H., Dohra, H., Moriwaki, K., Miyoshi, E., Hirabayashi, J., and Kawagishi. H. (2012) A novel core fucose-specific lectin from themushroom Pholiota squarrosa. J. Biol. Chem. 287, 33973–33982 [PMID : 22872641]
- Miyoshi, E., and Nakano, M. (2008) Fucosylated haptoglobin is a novel marker for pancreatic cancer: detailed analyses of oligosaccharide structures. Proteomics 8, 3257–3262 [PMID : 18646007]
Patent information: 2010-011446 Differential diagnosis for colon cancer Yuka Kobayashi, Eiji Miyoshi
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Nakayama, Kotarosumitomo,
Miyoshi, Eiji,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.2,5,2024 .
How to Cite this Work in Website:
Nakayama, Kotarosumitomo,
Miyoshi, Eiji,
(2014).
Core fucose-specific lectin Pholiota squarrosa lectin and its application to histochemistry.
Retrieved 2,5,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t176.
html source
Nakayama, Kotarosumitomo,
Miyoshi, Eiji,
(2014).
<b>Core fucose-specific lectin <em>Pholiota squarrosa</em> lectin and its application to histochemistry</b>.
Retrieved 5 2,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t176" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t176</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|