Ficolins are a group of oligomeric lectins with subunits consisting of collagen- and fibrinogen-like domains. Most ficolins identified to date recognize N-acetylglucosamine (GlcNAc). Three types of ficolins, L-ficolin, H-ficolin and M-ficolin are present in human serum. L-ficolin (also called “ficolin-2”) recognizes the acetyl group moiety present in substances such as GlcNAc. L-ficolin forms complexes with three types of serine protease (MASP-1, MASP-2 and MASP-3) and two types of their truncated form (sMAP and MAp44). Binding of the L-ficolin-MASP complex to carbohydrates on the surface of pathogens activates the lectin pathway of the complement system (an effector system of immunity). Of the three types of ficolins present in human serum, purification methods and binding assays have well been established for L-ficolin. |
| Category | Sugar binding proteins |
| Protocol Name | Isolation and binding assay of L-ficolin |
Authors
 |
Matsushita, Misao
Department of Applied Biochemistry, School of Engineering, Tokai University
|
| KeyWords |
|
Reagents
 |
| ● |
Pooled human plasma from healthy donors |
| ● |
|
| ● |
GlcNAc-agarose (Sigma-Aldrich, St. Louis, MO) |
| ● |
Mono Q HR5/5 (GE Healthcare, Little Chalfont, UK) |
| ● |
Loading buffer for GlcNAc-agarose affinity chromatography: 50 mM Tris, 200 mM NaCl, 10 mM CaCl2, pH 7.8 (buffer A) |
| ● |
Elution buffer for mannose-binding lectin (MBL, also called mannan-binding protein (MBP)) from GlcNAc-agarose: 50 mM Tris, 200 mM NaCl, 10 mM CaCl2, 300 mM mannose, pH 7.8 (buffer B) |
| ● |
Elution buffer for L-ficolin from GlcNAc-agarose: 50 mM Tris, 200 mM NaCl, 10 mM CaCl2, 300 mM GlcNAc, pH 7.8 (buffer C) |
| ● |
Loading buffer for Mono Q ion-exchange chromatography: 20 mM Tris, 50 mM NaCl, 10 mM CaCl2, pH 7.8 (buffer D) |
| ● |
Elution buffer for Mono Q ion-exchange chromatography: 20 mM Tris, 1M NaCl, 10 mM CaCl2, pH 7.8 (buffer E) |
| ● |
12% and 5% polyacrylamide gels, for SDS-PAGE under reducing and non-reducing conditions, respectively. |
| ● |
Acetylated low-density lipoprotein (Ac-LDL) (Harbor Bio-Products, Norwood, MA) |
| ● |
Microtiter plates: (Iwaki Glass Co., Ltd., Tokyo, Japan) |
| ● |
Carbonate buffer (0.1 M, pH 9.6) |
| ● |
TBS: 20 mM Tris, 150 mM NaCl, pH 7.5 |
| ● |
TBS-Ca-T: TBS containing 2 mM CaCl2, and 0.05 % Tween 20 |
| ● |
Blocking solution: StartingBlock Blocking Buffer (Thermo Fisher Scientific Inc., Waltham, MA) |
| ● |
Rabbit anti-L-ficolin polyclonal antibody: prepared in a lab (Fukushima Medical University, Fukushima, Japan) |
| ● |
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA) |
| ● |
Substrate solution: 3,3’,5,5´-tetramethylbenzidene (TMB) (Wako Pure Chemical Industries, Ltd.,Osaka, Japan) |
|
Instruments
 |
| ● |
Columns for open-column chromatography |
| ● |
Ultrafiltration membrane filter (cut-off, 30 kDa) equipped with a centrifugal unit: Amicon Ultra (Merck Millipore, Billerica, MA) |
| ● |
0.22 mM membrane filter (Merck Millipore) |
| ● |
|
|
| Methods |
|
1. |
|
| 1) |
Prepare 1 L pooled human plasma (starting material). |
Comment 0
|

|
| 2) |
Add 1 M CaCl2 to the plasma to a final concentration of 10 mM and incubate for 1 h at 37°C to allow clotting. |
Comment 0
|

|
| 3) |
Centrifuge the plasma at 10,000 × g for 30 min at 4°C and save the supernatant as the human serum. |
Comment 0
|

|
| 4) |
Add polyethylene glycol 4,000 to the serum to a final concentration of 8% and incubate for 1 h at 4°C. |
Comment 0
|

|
| 5) |
Centrifuge the plasma at 10,000 × g for 30 min at 4°C. |
Comment 0
|

|
| 6) |
Dissolve the precipitate in 200 mL of GlcNAc-agarose loading buffer (buffer A). |
Comment 0
|

|
| 7) |
Apply the solution to the GlcNAc-agarose column previously equilibrated with buffer A. |
Comment 0
|

|
| 8) |
Wash the column with approximately 10 column volumes of buffer A. |
Comment 0
|

|
| 9) |
Elute the bound components with buffer B. |
Comment 0
|

|
| 10) |
Elute the bound components with GlcNAc-agarose elution buffer for L-ficolin (buffer C). |
Comment 0
|

|
| 11) |
Concentrate the eluate from GlcNAc agarose with buffer C by centrifugation in (cutoff 30 kDa ) Amicon Ultra to approximately 8 mL. |
Comment 0
|

|
| 12) |
Dialyze the concentrated fraction against loading buffer for Mono Q (buffer D). |
Comment 0
|

|
| 13) |
Apply the dialysate, being passed through a 0.22-mm membrane filter, to Mono Q previously equilibrated with buffer D. |
Comment 0
|

|
| 15) |
Elute the bound components with a linear gradient from 50 mM NaCl (100 % buffer D) to 0.5 M NaCl (50% buffer E). L-ficolin is eluted at a concentration between 0.15 NaCl and 0.2 M NaCl. |
Comment 0
|

|
| 16) |
Subject L-ficolin to SDS-PAGE under reducing (12% gel) or non-reducing (5% gel) conditions. |
Comment 0
|
|
|
|
2. |
Binding assay of L-ficolin |
| 1) |
Coat a microtiter plate with 10 μg/mL Ac-LDL in carbonate buffer (100 μL/well) for 18 h at 4°C. |
Comment 0
|

|
| 2) |
Block with blocking solution for 2 h at 37°C. |
Comment 0
|

|
| 4) |
Incubate the wells for 1 h at room temperature with L-ficolin (100 μL/well) diluted with TBS-Ca-T. |
Comment 0
|

|
| 6) |
Add rabbit anti-L-ficolin in TBS-Ca-T (100 μL/well) to the wells. |
Comment 0
|

|
| 7) |
Incubate for 1 h at room temperature. |
Comment 0
|

|
| 9) |
Add HRP-conjugated-anti-rabbit IgG in TBS-Ca-T (100 μL/well) to the wells. |
Comment 0
|

|
| 10) |
Incubate for 1 h at room temperature. |
Comment 0
|

|
| 12) |
Add TMB (100 μL/well) to the wells. |
Comment 0
|

|
| 13) |
Incubate for 5–20 min at room temperature. |
Comment 0
|

|
| 14) |
Add 1N HCl (100 μL/well) to the wells. |
Comment 0
|

|
| 15) |
Measure absorbance at 450 nm using a microplate reader. |
Comment 0
|
|
|
| Notes | Isolation of L-ficolin
On the GlcNAc-agarose column, MBL is first eluted with mannose (buffer B), followed by the elution of L-ficolin with GlcNAc (buffer C). The difference in the elution profiles between these human serum lectins is because of their binding specificities. MBL binds mannose and GlcNAc, whereas L-ficolin binds GlcNAc but not mannose. Calcium ions are included in the buffers throughout the purification steps. This is because L-ficolin forms a complex with MASP-1, MASP-2, MASP-3, sMAP, and MAp44 in a calcium-dependent manner in human serum. Therefore, the L-ficolin preparation purified according to this method contains these L-ficolin-associated proteins. Approximately 100 μg of the L-ficolin complex is obtained from 1 L of human serum.
Binding assay of L-ficolin
L-ficolin recognizes the acetyl group in a calcium-independent manner, and therefore, can bind to Ac-LDL in the wells. A buffer containing calcium ions (TBS-Ca) is used in the present method, because L-ficolin used in the present binding assay forms complexes with its associated proteins as described above. These conditions allow us to assay the activation of the lectin pathway mediated by the L-ficolin complex bound to Ac-LDL. When assaying L-ficolin binding to Ac-LDL, buffers such as TBS and PBS without calcium ions can also be used. Antibodies including peptide and monoclonal ones against L-ficolin are commercially available. |
| Figure & Legends |
Figure & Legends 
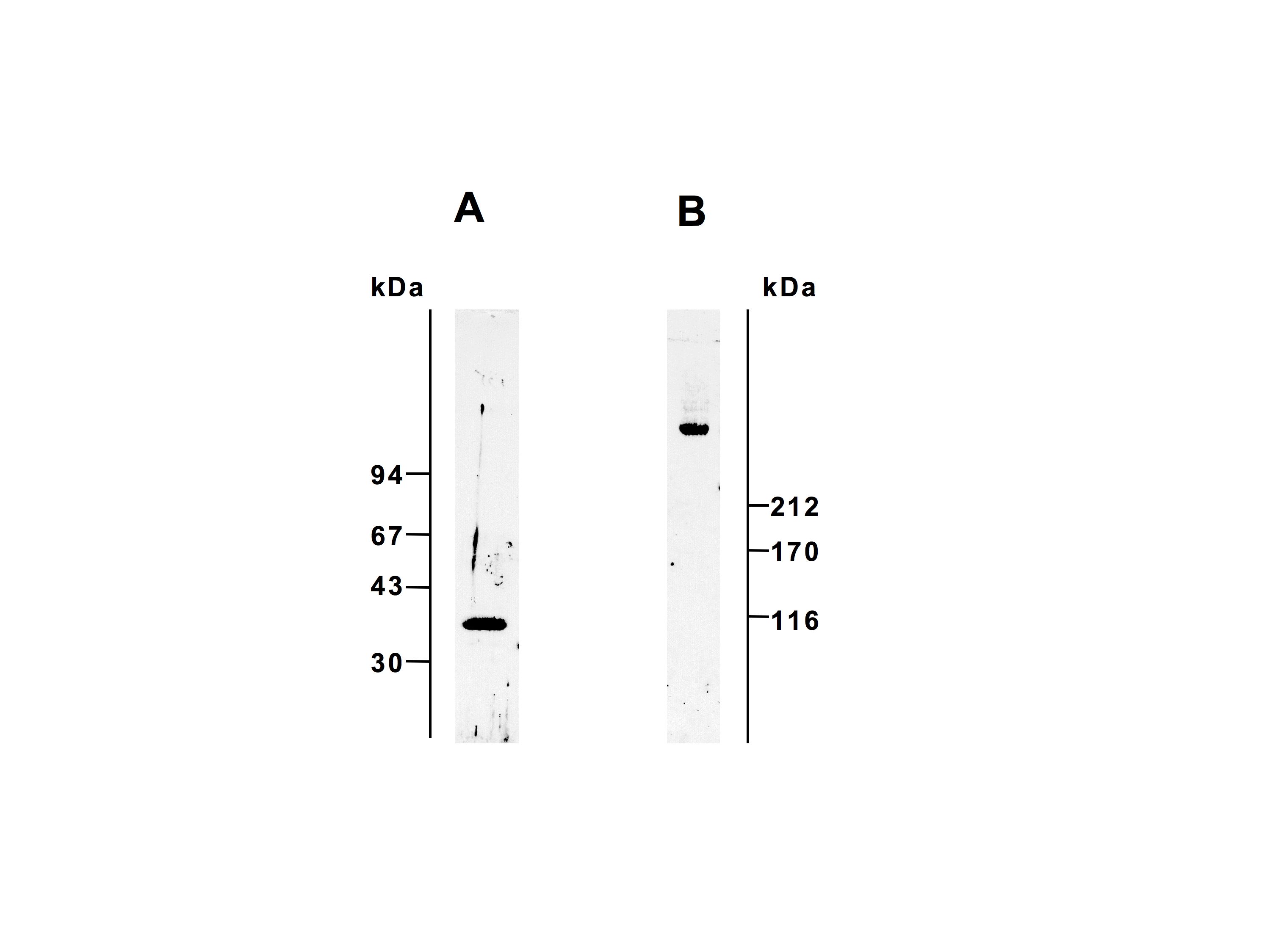
Fig. 1. SDS-PAGE profiles of L-ficolin.
L-ficolin was subjected to SDS-PAGE under reducing (A, 12 % gel) and non-reducing (B, 5 % gel) conditions. The proteins were stained with Coomassie Brilliant Blue R-250.

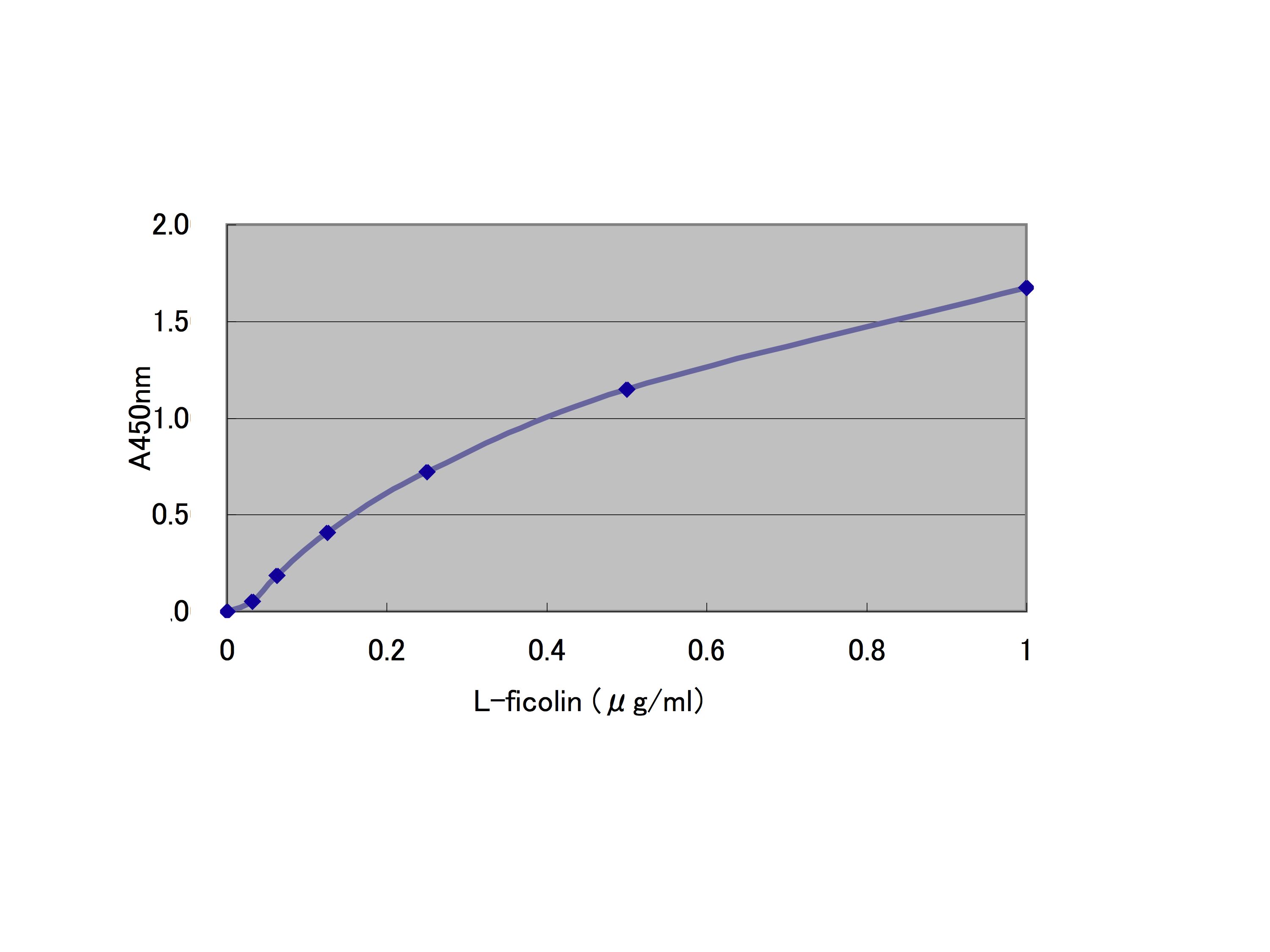
Fig. 2. L-ficolin binding to Ac-LDL |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-10-24 14:33:48 |
- Matsushita, M., Endo, Y., Taira, S., Sato, Y., Fujita, T., Ichikawa, N., Nakata, M. and Mizuochi, T. (1996) A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J. Biol. Chem. 271, 2448–2454 [PMID : 8576206]
- Matsushita, M. (2009) Ficolins: complement-activating lectins involved in innate immunity J. Innate Immun. 2, 24–32 [PMID : 20375620]
- Matsushita, M., Endo, Y. and Fujita, T. (2000) Cutting edge: Complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J. Immunol. 164, 2281–2284 [PMID : 10679061]
- Faro J., Chen Y., Jhaveri P., Oza P., Spear GT., Lint TF. and Gewurz H. (2008)L-ficolin binding and lectin pathway activation by acetylated low-density lipoprotein. Clin. Exp. Immunol. 151, 275–283 [PMID : 18031558]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Matsushita, Misao,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.30,4,2024 .
How to Cite this Work in Website:
Matsushita, Misao,
(2014).
Isolation and binding assay of L-ficolin.
Retrieved 30,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t101.
html source
Matsushita, Misao,
(2014).
<b>Isolation and binding assay of L-ficolin</b>.
Retrieved 4 30,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t101" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t101</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|