The cellular localization as well as tissue distribution of heparin sulfate (HS) can be investigated by immunostaining using anti-HS antibodies. However, it should be noted that HS preparations derived from various sources differ in their reactivity to an anti-HS antibody depending on the epitope content along the HS chain. A single anti-HS antibody can react with only particular HS chains which have a specific epitope structure. To detect all the HS-proteoglycans simultaneously, the anti-HS “stub” antibody, F69-3G10, is available after treatment with bacterial heparin/HS lyases. The epitope structure of this antibody is generated only by digestion with the bacterial lyases. Characteristics of major anti-HS antibodies are summarized in “Characteristics and epitopes for anti-heparan sulfate antibodies”. |
| Category | Glycosaminoglycans |
| Protocol Name | Application of anti-GAG antibody and biotinylated hyaluronan binding protein(bHABP) [3] ~ Immunostaining using anti-heparan sulfate antibody |
Authors
 |
Kaneiwa, Tomoyuki
Laboratory of Proteoglycan Signaling and Therapeutics, Faculty of Advanced Life Science, Hokkaido University
Kogawa, Ryo
Laboratory of Proteoglycan Signaling and Therapeutics, Faculty of Advanced Life Science, Hokkaido University
Yamada, Shuhei
*
Department of Pathobiochemistry, Faculty of Pharmacy, Meijo University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
0.1 M Phosphate-buffered saline (PBS) |
| ● |
|
| ● |
Bovine serum albumin (BSA) |
| ● |
Anti-HS antibody, F58-10E4 (Seikagaku Corp., Tokyo, Japan) |
| ● |
Alexa Fluor® 488 goat anti-mouse IgM, 2 mg/mL (Molecular Probes, Eugene, OR) |
| ● |
Anti-mouse IgM biotin conjugate antibody |
| ● |
4’,6-Diamino-2-phenylindole (DAPI) (Invitorogen/Life Technologies, Carlsbad, CA) |
| ● |
VECTASHIELS mounting medium (Funakoshi Co., Ltd., Tokyo, Japan) |
| ● |
|
| ● |
Vectastain ABC kit (Vector Laboratories Inc., Burlingame, CA) |
| ● |
3-3’-Diaminobenzidine (DAB) tetrahydrochloride |
|
Instruments
 |
| ● |
Fluorescence microscope, BZ-9000 (KEYENCE Corporation, Osaka, Japan) |
|
| Methods |
|
1. |
|
| 1) |
Seed cells on a cover glass in a 6-cm dish. |
Comment 0
|

|
| 2) |
Transfer the cover glass to a 6-well plate and wash it with PBS three times. |
Comment 0
|

|
| 3) |
Add 1 mL of 4% PFA/PBS to the well for fixing and incubate at room temperature for 30 min. |
Comment 0
|

|
| 4) |
Wash the cover glass with PBS for one second once and then for 5 min three times. |
Comment 2
|

|
| 5) |
Add 200 μL of 1% BSA/PBS to the cover glass for blocking and incubate at room temperature for 30 min. |
Comment 0
|

|
| 6) |
Wash the cover glass with PBS two times and then with 1% BSA/PBS once. |
Comment 0
|

|
| 7) |
Transfer the cover glass to a humid box. |
Comment 2
|

|
| 8) |
Add 200 μL of the anti-HS antibody F58-10E4 (diluted 1:200 in 1% BSA/PBS), and incubate at 4oC overnight. |
Comment 0
|

|
| 9) |
Transfer the cover glass to a 6-well plate and wash it with PBS for 5 min three times. |
Comment 0
|

|
| 10) |
Wash the cover glass with 1% BSA/PBS two times. |
Comment 1
|

|
| 11) |
Transfer the cover glass to a humid box. |
Comment 2
|

|
| 12) |
Add 200 μL of the secondary antibody, Alexa Fluor® 488 goat anti-mouse IgM (diluted 1: 200 in 1% BSA/PBS), and incubate for 1 h at room temperature with light shielding. |
Comment 0
|

|
| 13) |
Transfer the cover glass to a 6-well plate and wash it with PBS for 5 min three times. |
Comment 0
|

|
| 14) |
Add 1 mL of 5 ng/mL DAPI and incubate for 5 min at room temperature with light shielding. |
Comment 0
|

|
| 15) |
Wash the cover glass with PBS for one second once and then for 5 min three times. |
Comment 2
|

|
| 16) |
Mount the cover glass using the mounting medium. |
Comment 0
|

|
| 17) |
Capture images with a fluorescent microscope (Fig. 1). |
Comment 0
|
|
|
|
2. |
|
| 1) |
Soak a paraffin-embedded section on a glass slide in 100% (twice), 90%, 70%, and 50% ethanol sequentially for 5 min each, and then in 2 changes of H2O for 5 min each in a Coplin jar at room temperature for deparaffinization and hydration. |
Comment 1
|

|
| 2) |
Soak the section in 2 changes of PBS for 5 min and then 0.1% Triton X-100/PBS for 10 min for retrieval of the antigen. |
Comment 0
|

|
| 3) |
Soak the slide in 2 changes of PBS and then in H2O for 5 min. |
Comment 0
|

|
| 4) |
Soak the slide in 3% H2O2/methanol at room temperature for 30 min for inactivation of endogenous peroxidases. |
Comment 0
|

|
| 5) |
Soak the slide in 2 changes of H2O and then 2 changes of PBS for 5 min each. |
Comment 0
|

|
| 6) |
Add 200 μL of 3% BSA/PBS to the section for blocking in a humid box and incubate for 1 h at room temperature. |
Comment 0
|

|
| 7) |
Tap off the excess BSA/PBS solution. |
Comment 0
|

|
| 8) |
Add 200 μL of the anti-HS antibody, F58-10E4 (diluted 1:100 in 1% BSA/PBS), to the section in a humid box and incubate at 4oC overnight. |
Comment 0
|

|
| 9) |
Tap off the excess antibody solution. |
Comment 0
|

|
| 10) |
Soak the slide in PBS for one second once and then in 3 changes of PBS for 5 min each. |
Comment 2
|

|
| 11) |
Add 200 μL of the secondary antibody, anti-mouse IgM biotin conjugate antibody (diluted 1:100 in 1% BSA/PBS), to the section in a humid box and incubate at room temperature for 1 h. |
Comment 0
|

|
| 12) |
Tap off the excess secondary antibody solution |
Comment 0
|

|
| 13) |
Soak the slide in PBS for one second once and then in 3 changes of PBS for 5 min each |
Comment 0
|

|
| 14) |
Add 200 μL of the ABC solution of the Vectastain ABC kit (diluted 1:200 in PBS) to the section in a humid box and incubate at room temperature for 1 h |
Comment 0
|

|
| 15) |
Tap off the excess ABC solution. |
Comment 0
|

|
| 16) |
Soak the slide in PBS for one second once and then in 3 changes of PBS for 5 min each. |
Comment 2
|

|
| 17) |
Soak the slide in 0.06% DAB/PBS for 10 min and then in 0.01% H2O2/0.06% DAB/PBS within 10 min until the color is developed. |
Comment 0
|

|
| 18) |
Soak the slide in PBS for one second to terminate the reaction and then in 3 changes of PBS for 5 min each. |
Comment 0
|

|
| 19) |
Soak the slide in H2O for 5 min and then in 20%, 40%, 80%, and 100% ethanol sequentially for 5 min each. |
Comment 0
|

|
| 20) |
Soak the slide in 3 changes of xylene for 10 min each. |
Comment 0
|

|
| 22) |
Mount the sections under a cover glass using the mounting medium. |
Comment 0
|

|
| 23) |
Capture images with a microscope. |
Comment 0
|
|
|
| Figure & Legends |
Figure & Legends

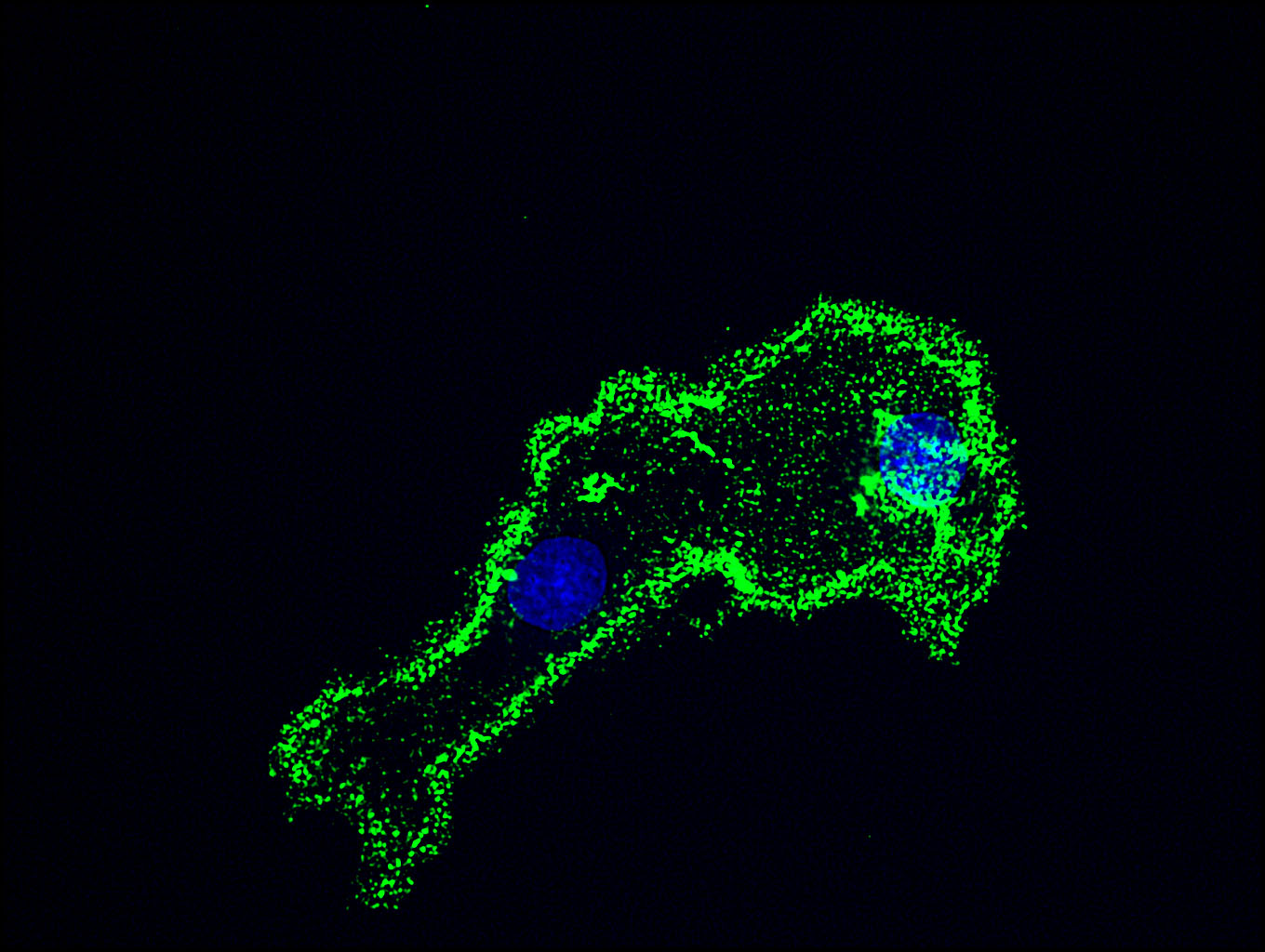
Fig. 1. The immunostaining of COS-7 cells for HS using F58-10E4.
Strong staining (green) was observed at the periphery of the cells. DAPI stains the nucleus (blue).

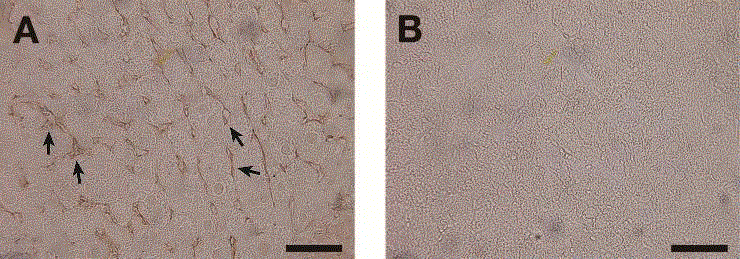
Fig. 2. Sections of liver stained with the anti-HS antibody F58-10E4.
A significant staining with F58-10E4 (A) was observed between hepatic cells (indicated by arrows), but not with normal mouse IgM (negative control) (B). Scale bar, 50 μm. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2017-01-23 15:53:15 |
- Li, J., Kleeff, J., Abiatari, I., Kayed, H., Giese, N.A., Felix, K., Giese, T., Büchler, M.W., and Friess, H. (2005) Enhanced levels of Hsulf-1 interfere with heparin-binding growth factor signaling in pancreatic cancer. Mol. Cancer 4, 14 [PMID : 15817123]
- Stickens, D., Zak, B.M., Rougier, N., Esko, J.D., and Werb, Z. (2005) Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 132, 5055-5068 [PMID : 16236767]
- Pan, Y., Woodbury, A., Esko, J.D., Grobe, K., and Zhang, X. (2006) Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133, 4933-4944 [PMID : 17107998]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Kaneiwa, Tomoyuki,
Kogawa, Ryo,
Yamada, Shuhei,
(2017). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Kaneiwa, Tomoyuki,
Kogawa, Ryo,
Yamada, Shuhei,
(2017).
Application of anti-GAG antibody and biotinylated hyaluronan binding protein(bHABP) [3] ~ Immunostaining using anti-heparan sulfate antibody.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t165.
html source
Kaneiwa, Tomoyuki,
Kogawa, Ryo,
Yamada, Shuhei,
(2017).
<b>Application of anti-GAG antibody and biotinylated hyaluronan binding protein(bHABP) [3] ~ Immunostaining using anti-heparan sulfate antibody</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t165" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t165</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|