Galectin-1 is a multi-modal lectin which functions in a wide variety of physiological contexts. We reported that Galectin-1 is expressed in neural stem cells (NSCs) and promotes their proliferation in the subventricular zone in adult mouse brain (Sakaguchi et al., 2006, Sakaguchi et al., 2010). Furthermore, we showed that this activity of Galectin-1 is essential for its ameliorating effects in the functional deficits caused by brain ischemia in gerbil (Ishibashi et al., 2007) and by spinal cord injury in non-human primate (Yamane et al., 2010). In this protocol, we will present several methods that can be used to study Galectin-1 in adult NSCs and potentially make a fundamental contribution to the research field. The methods include: 1) Culturing NSCs in vitro with Galectin-1, 2) Expression analysis of Galectin-1 in the adult NSCs, 3) Infusion of Galectin-1 into the lateral ventricle of adult mice brain and quantification of its effect on adult NSCs. Although the protocols are optimized for use in neuronal cells or tissue samples from mouse central nervous system, they can be easily applied to other cell-types or tissues derived from other organisms. |
| Category | Sugar binding proteins |
| Protocol Name | Recombinant sugar binding proteins and their functional analysis: Galectin-1 |
Authors
 |
Sakaguchi, Masanori
Department of Physiology, School of Medicine, Keio University
Sawamoto, Kazunobu
Department of Physiology, School of Medicine, Keio University
Okano, Hideyuki
*
Department of Physiology, School of Medicine, Keio University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Neurosphere Culture Medium
- DMEM/F12 1:1 (Sigma-Aldrich, St. Louis, MO)
- Glucose (Sigma-Aldrich)
- Transferrin (Sigma-Aldrich)
- Inslin (Sigma-Aldrich)
- Progesterone (Sigma-Aldrich)
- Sodium Selenate (Sigma-Aldrich)
- Putrescine (Sigma-Aldrich)
- EGF (Genzyme Corporation, Cambridge, MA)
- FGF (Genzyme Corporation)
- B27 (Invitrogen/Life Technologies, Inc., Carlsbad, CA)
- HEPES (Sigma-Aldrich)
|
| ● |
Functional assay using Galectin-1 in vitro
- rGalectin-1 (Genzyme Corporation)
- TDG (Sigma-Aldrich)
- CS-Galectin-1 (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan)
- CS-Galectin-1 (Hirabayashi and Kasai, 1991)
|
| ● |
Cell dissociation
- Accumax (Innovative Cell Technologies, Inc., San Diego, CA)
- DNase (Roche, Basel, Switzerland)
|
| ● |
Immunohistochemistry
- anti-Galectin-1 antibody Goat polyclonal (AF1245, R&D systems Inc., Minneapolis, MN)
- anti-Galectin-1 antibody Rabbit polyclonal (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan)
- 24-well plastic dishes plate (Corning, Corning, NY)
- TSA kit (PerkinElmer, Waltham, MA)
- Tris-NaCl-blocking (TNB) blocking buffer (Comes with TSA kit)
- ABC Elite kit (Vector Laboratories Inc., Burlingame, CA)
- Secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA)
- Alexa Fluor-conjugated secondary antibodies (Molecular Probes, Eugene, OR)
- Hoechst 33258 (Sigma-Aldrich)
- Permafluor (Thermo Fisher Scientific Inc., Waltham, MA)
|
| ● |
Infusion into the lateral ventricle
- CS-Galectin-1 (Kyowa Hakko Kirin Co., Ltd.)
- CS-Galectin-1 (Hirabayashi and Kasai, 1991)
|
|
Instruments
 |
| ● |
Vibratome VT1200S (Leica Microsystems GmbH, Watzlar, Germany) |
| ● |
Stereotaxic Surgery device (Muromachi Kagaku Kougyo, Inc., Seki, Japan) |
| ● |
Osmotic infusion pump (ALZET Osmotic Pumps, Cupertino, CA) |
| ● |
28-gauge internal cannula (Unique Medical Co., Ltd., Tokyo, Japan) |
| ● |
Laser Scan Microscope (LSM510)(Carl Zeiss AG, Jena, Germany) |
|
| Methods |
|
1. |
Culturing neural stem cells (NSCs) in vitro with Galectin-1 |
| 1) |
Day 1
Isolate subventricular zone tissue from E14.5 embryos and place in ice-cold culture media until all the embryos required are dissected. |
Comment 1
|

|
| 2) |
Add 3 mL of Accumax (supplemented with DNAse at 10 μg/mL), incubate in a 37°C water bath for 10 min, and then dissociate cell aggregates by pipetting up and down with a 5 mL pipette for about 10 times. |
Comment 1
|

|
| 3) |
Wash the tissue by adding 10 mL of culture media and centrifuge at 800 rpm (140 g) for 5 min. |
Comment 0
|

|
| 4) |
Re-suspend the pellet in culture media and perform cell count and viability test (e.g. trypan blue exclusion assay). Viability is usually > 70% (if lower, abort the experiment and repeat from step 1). |
Comment 0
|

|
| 5) |
Dilute viable cells in culture media to a density of 0.5–1×106cells/mL and incubate at 37°C, 5% CO2 (the cells should be mostly discrete single cells) |
Comment 0
|

|
| 6) |
Day 4
Add the same volume of culture media as used for plating so that the original volume is doubled. |
Comment 1
|

|
| 7) |
Day7
Passage the neurospheres (Fig. 1) using Accumax as day 1. At this time point, EGF and FGF-2 responsive NSCs are highly condensed among the cultured cells. Add Galectin-1 to the media immediately after plating the cells at desired concentration. 2ME (10mM) should also be added to maintain Galectin-1's carbohydrate binding activity. |
Comment 1
|
|
|
|
2. |
Expression analysis of Galectin-1 in the adult NSCs |
| 1) |
Day 1
Brains are perfusion-fixed with 4% paraformaldehyde and post-fixed in the same fixative overnight. |
Comment 1
|

|
| 2) |
Day 2
Brains are cut into 50 mm sections using a vibratome. Brain Matrix (see above) is useful to trim the brain to mount on the vibratome. Place the sections into a 24 well plate filled with 60%Glycerol/ PBS/ 0.1% NaN3 solution and keep at 4°C overnight. On the next day, transfer the plate to a −20°C freezer until required. The sections can be stored at 20°C for over 5 years. |
Comment 1
|

|
| 3) |
Rinse the sections three times in PBS/ 3%H2O2 and then place into citric acid buffer (described above). Heat the sections to 95°C for 1–4 min using a microwave. |
Comment 1
|

|
| 4) |
Rinse the sections three times in PBS. Incubate with anti-Galectin-1 primary antibody (see above) in TNB buffer overnight at 4°C. For double or triple immunolabeling, other primary antibodies can also be applied at this stage. |
Comment 1
|

|
| 5) |
Day 3
Wash the sections three times in PBS. Incubate with HRP-conjugated anti-rabbit IgG antibody (see above) and a nuclear dye (e.g., Hoechst 333258) in TNB buffer (see above) for 60 min at room temperature. |
Comment 1
|

|
| 6) |
Rinse the sections three times in PBS and perform TSA reaction (see above, also refer to the kit’s instructions) for 10 min. |
Comment 0
|

|
| 7) |
Mount sections onto a glass slide using Permafluor (see above). The sections are now ready for examination and analysis using fluorescence microscopy (Fig. 2). |
Comment 1
|
|
|
|
3. |
Infusion of Galectin-1 into the lateral ventricle of adult mouse brain and quantification of its effect on adult NSCs. |
| 1) |
Anesthetize the mouse with a mixture of gas (N2 3.5 L/min, O2 1.5 L/min, Isoflurane 2 L/min). |
Comment 1
|

|
| 2) |
Implant a 28-gauge guide cannula into the lateral ventricle using the following coordinates: 0.2 mm anterior from the bregma, 0.8 mm lateral from the midline, and 2.0 mm deep from the brain surface. |
Comment 0
|

|
| 3) |
Load the micropump injector with 56 μL of solution containing CS-Galectin-1 (10 mg/mL) in PBS. Gradually infuse the solution into the lateral ventricle of the mouse brain for 7 days by osmotic pressure. |
Comment 1
|

|
| 4) |
To label adult NSCs, administer BrdU (1 mg/mL) to the animal’s drinking water during the period of infusion (7 days). |
Comment 1
|

|
| 5) |
The mouse is sacrificed 10 days after the last day of the infusion and the brain is processed for immunohistochemistry and counting (Fig. 3). |
Comment 1
|
|
|
| Notes | Because of space limitations, detailed procedures for stereotaxic surgery are omitted from this article. However further information can be found in the reference (Athos and Strom, 2001). |
| Figure & Legends |
Figure & Legends 
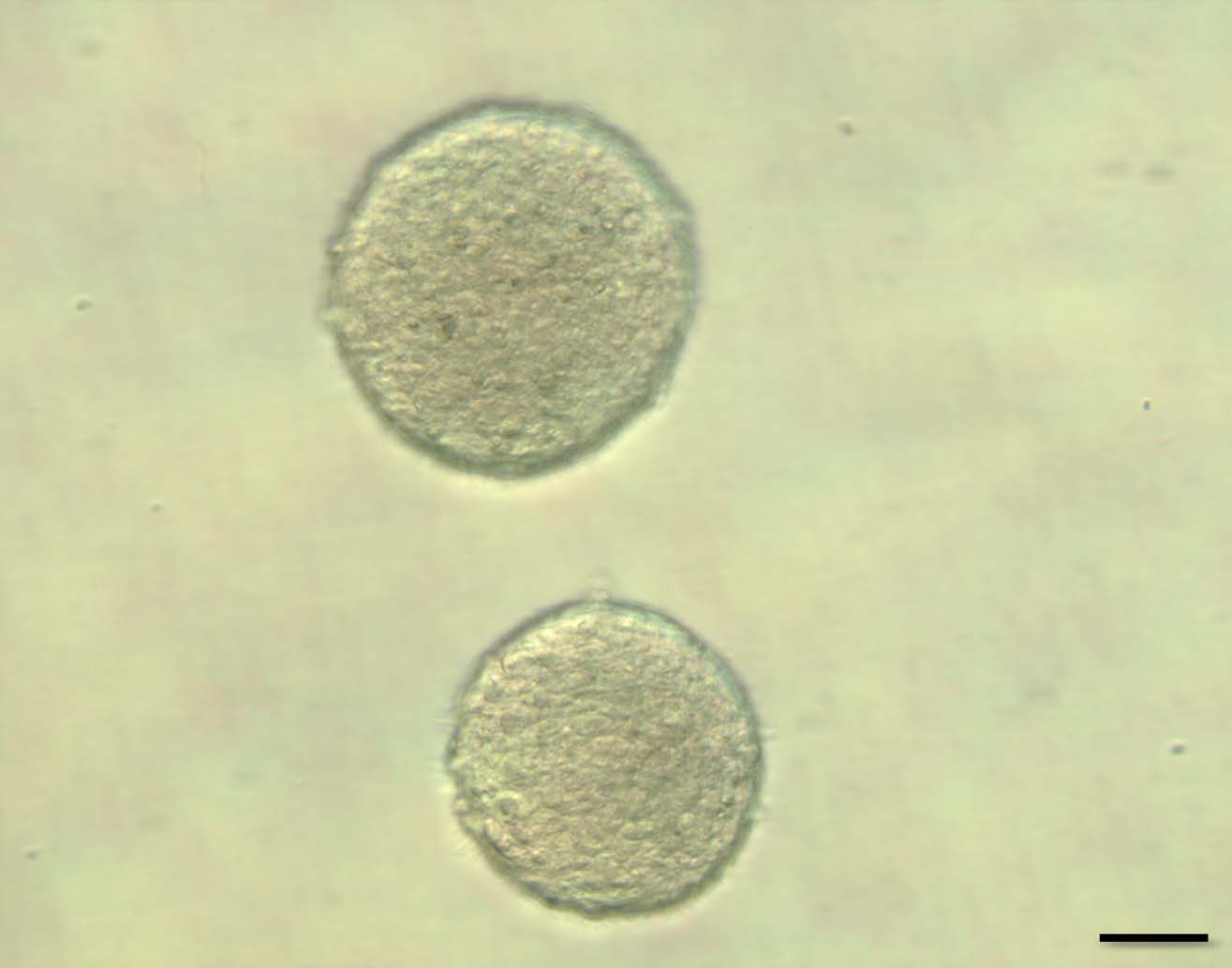
Fig. 1
Neurospheres were formed after 7-day culture of the subventricular zone cells in vitro. The neurosphere which contains NSCs is usually over 100 μm in its diameter. The neurosphere is dissociated to single cells, Galectin-1 is added in the culture, and the efficiency of the secondary neurosphere formation is evaluated compared with appropriate control culture. Scale bar = 50 μm.

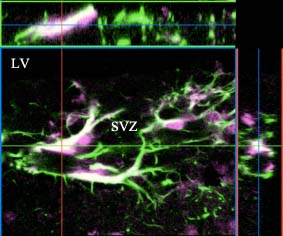
Fig. 2
Galectin-1 (purple) and a NSC marker, GFAP (green), were colabeled in the subventricular zone. Laser scan microscopic analysis indicates the colocalization of the two signals (white).

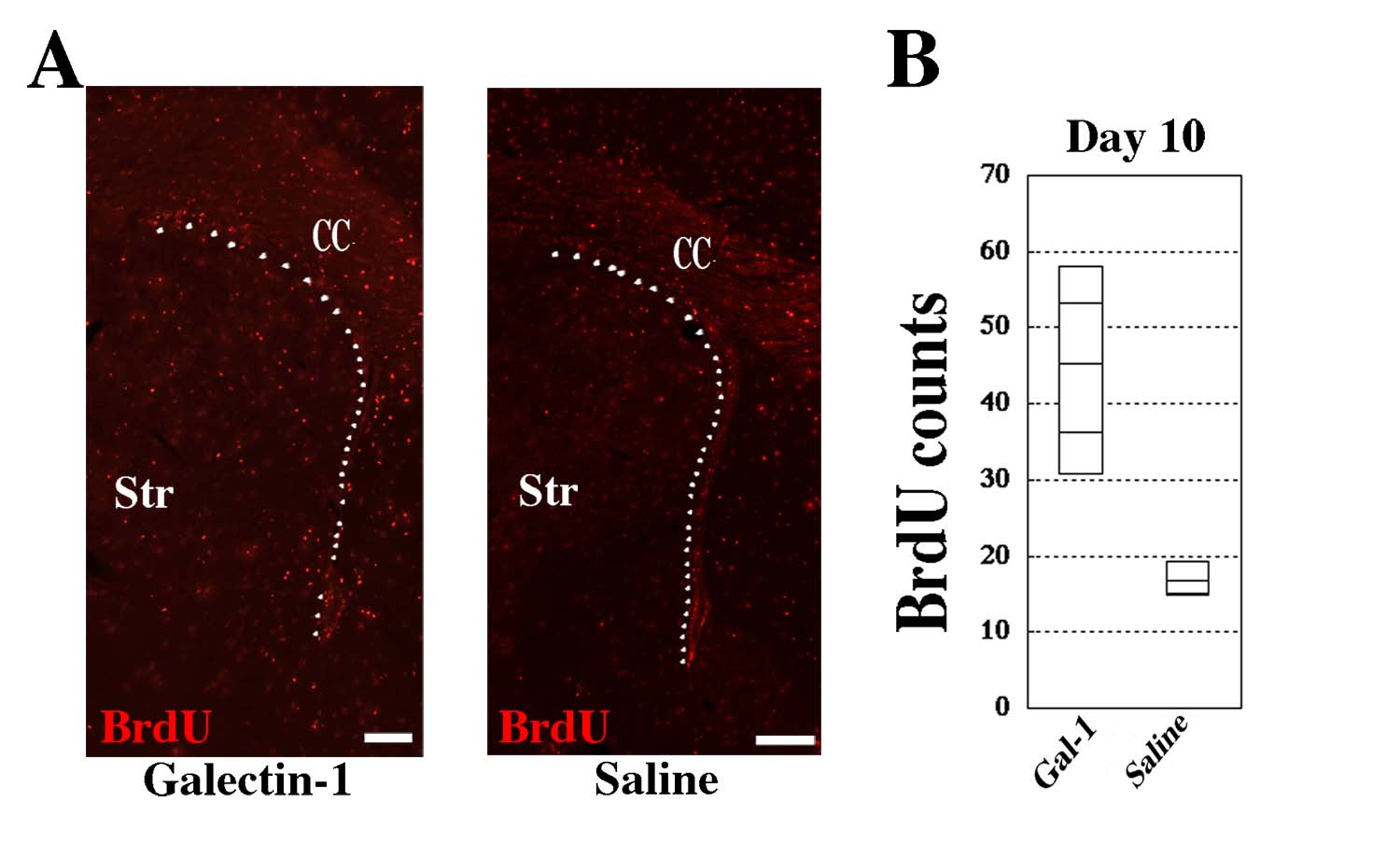
Fig. 3
(A) BrdU signals were visualized by immunohistochemistry in the subventricular zones (indicated by white dots) of Galectin-1 infused (left) and Saline infused (right) mice brains. (B) BrdU-positive cells were significantly increased in the subventricular zone of the Galectin-1 infused brain 10 days after the last infusion. The effect was more prominent when the CS-Galectin-1 is infused (Sakaguchi et al., 2006). CC, Corpus Callosum; Str, Striatum. Scale bar = 200 μm. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-07-31 09:38:30 |
- Sakaguchi, M., Imaizumi, Y., Shingo, T., Tada, H., Hayama, K., Yamada, O., Morishita, T., Kadoya, T., Uchiyama, N., Shimazaki, T., Kuno, A., Poirier, F., Hirabayashi, J., Sawamoto, K., and Okano, H. (2010) Regulation of adult neural progenitor cells by Galectin-1/β1 Integrin interaction. J Neurochem. 113, 1516–1524 [PMID : 20367753]
- Yamane, J., Nakamura, M., Iwanami, A., Sakaguchi, M., Katoh, H., Yamada, M., Momoshima, M., Miyao, S., Ishii, K., Tamaoki, N., Nomura, T., Okano, H.J., Kanemura, Y., Toyama, Y., and Okano, H. (2010) Transplantation of Galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. Journal of Neuroscience Research. 88, 1394–1405 [PMID : 20091712]
- Ishibashi, S., Kuroiwa, T., Sakaguchi, M., Sun, L., Kadoya, T., Okano, H., and Mizusawa, H. (2007) Galectin-1 regulates neurogenesis in the subventricular zone and promotes functional recovery after stroke. Exp Neurol. 207, 302–313 [PMID : 17706645]
- Sakaguchi, M., Shingo, T., Shimazaki, T., Okano, H.J., Shiwa, M., Ishibashi, S., Oguro, H., Ninomiya, M., Kadoya, T., Horie, H., Shibuya, A., Mizusawa, H., Poirier, F., Nakauchi, H., Sawamoto, K., and Okano, H. (2006) A carbohydrate binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA 103, 7112–7117 [PMID : 16636291]
- Athos, J., and Storm, D.R. (2001) High precision stereotaxic surgery in mice. Curr Protoc Neurosci. May;Appendix 4
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Sakaguchi, Masanori,
Sawamoto, Kazunobu,
Okano, Hideyuki,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.7,4,2025 .
How to Cite this Work in Website:
Sakaguchi, Masanori,
Sawamoto, Kazunobu,
Okano, Hideyuki,
(2014).
Recombinant sugar binding proteins and their functional analysis: Galectin-1.
Retrieved 7,4,2025 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t104.
html source
Sakaguchi, Masanori,
Sawamoto, Kazunobu,
Okano, Hideyuki,
(2014).
<b>Recombinant sugar binding proteins and their functional analysis: Galectin-1</b>.
Retrieved 4 7,2025 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t104" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t104</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|