
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002897 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002897

|
|||||||
Regist Date |
2012/06/29 18:35:06 | |||||||
| REACTANT | ||||||||
|
|
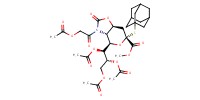
|
|||||||
|
|
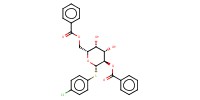
|
|||||||
|
|
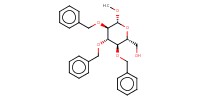
|
|
||||||
|
|
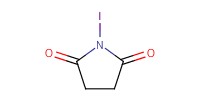
|
|||||||
Reactant Type |
NIS | |||||||
|
|

|
|||||||
Reactant Type |
TfOH | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
|
||||||
Yield |
55% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified, NOT specified | |||||||
Reaction Temp |
-78 degree C, -78 degree C | |||||||
Solvent |
CH2Cl2/CH3CN = 2/1, CH2Cl2/CH3CN = 2/1 | |||||||
Comment |
1) 87+88, NIS, TfOH, 2) +89, NIS, TfOH | |||||||
| Very few were described regarding this reaction. | ||||||||
| Acid-washed MS was included in the solvent. | ||||||||
| NIS and TfOH were added twice. | ||||||||
| COMMENT | ||||||||
| Keywords: diastereoselective synthesis, critical interplay, 2-deoxy-beta-glycopyranosides, beta-mannopyranosides, alpha-sialosides, alpha-glucopyranosides, beta-arabinofuranosides | ||||||||
| There are multiple phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000373 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo2017026 | |||||||
PubMed ID |
21919522 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (22): 9193-209. | |||||||
Article Title |
Methodology development and physical organic chemistry: a powerful combination for the advancement of glycochemistry. | |||||||
Author |
David, Crich | |||||||
Affiliation |
Department of Chemistry, Wayne State University, Detroit, Michigan 48202, USA. dcrich@chem.wayne.edu | |||||||
Reference Id |
REF-0000-000374 | |||||||
Source |
J. Org. Chem. 2011, 76, 9193-9209 | |||||||
Doi |
10.1021/jo2017026 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|