
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002892 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002892

|
||||
Regist Date |
2012/06/29 18:34:08 | ||||
| REACTANT | |||||
|
|
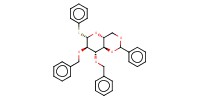
|
||||
|
|
|
||||
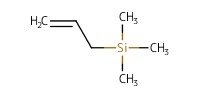
Reactant Type |
allyltrimethylsilane | ||||
|
|
|
||||
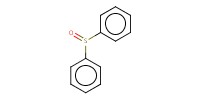
Reactant Type |
Ph2SO | ||||
|
|
|
||||
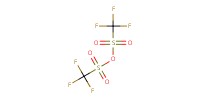
Reactant Type |
Tf2O | ||||
| PRODUCT | |||||
MOLECULE ID |
|
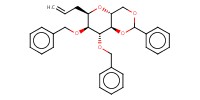
|
|||
Yield |
54% | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified, NOT specified | ||||
Reaction Temp |
-60 degree C, NOT specified | ||||
Solvent |
CH2Cl2, NOT specified | ||||
Comment |
1) +all except allyltrimethylsilane, 2) +allyltrimethylsilane | ||||
| Very few were described regarding this reaction. | |||||
| COMMENT | |||||
| Keywords: diastereoselective synthesis, critical interplay, 2-deoxy-beta-glycopyranosides, beta-mannopyranosides, alpha-sialosides, alpha-glucopyranosides, beta-arabinofuranosides | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000373 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo2017026 | ||||
PubMed ID |
21919522 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (22): 9193-209. | ||||
Article Title |
Methodology development and physical organic chemistry: a powerful combination for the advancement of glycochemistry. | ||||
Author |
David, Crich | ||||
Affiliation |
Department of Chemistry, Wayne State University, Detroit, Michigan 48202, USA. dcrich@chem.wayne.edu | ||||
Reference Id |
REF-0000-000374 | ||||
Source |
J. Org. Chem. 2011, 76, 9193-9209 | ||||
Doi |
10.1021/jo2017026 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|