
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002863 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002863

|
|||||||
Regist Date |
2012/06/21 20:00:52 | |||||||
| REACTANT | ||||||||
|
|
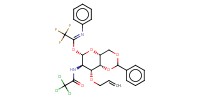
|
|
||||||
Mol |
0.25 mmol | |||||||
|
|
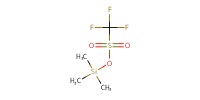
|
|||||||
Reactant Type |
TMSOTf (10% in CH2Cl2) | |||||||
Mol |
0.01 mmol | |||||||
|
|
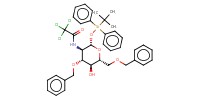
|
|
||||||
Mol |
0.20 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
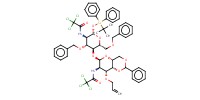
|
||||||
Product Type |
alpha | |||||||
Yield |
3% | |||||||
MOLECULE ID |
|
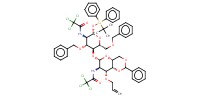
|
||||||
Product Type |
beta | |||||||
Yield |
94% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
1 hour, 1.5 hours | |||||||
Reaction Temp |
-78 degree C, -40 degree C | |||||||
Solvent |
anhydrous CH2Cl2 (6mL), anhydrous CH2Cl2 (6mL) | |||||||
Comment |
1) +all, 2) temperature change | |||||||
| MS AW300 was included in the solvent. | ||||||||
| COMMENT | ||||||||
| Keywords: Biantennary, N-glycans, LacNAc, LacdiNAc, SPPS | ||||||||
| There are multiple phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000371 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo200149d | |||||||
PubMed ID |
21612260 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (13): 5229-39. | |||||||
Article Title |
Synthesis of biantennary complex-type nonasaccharyl asn building blocks for solid-phase glycopeptide synthesis. | |||||||
Author |
Masashi, Hagiwara; Mizuki, Dohi; Yuko, Nakahara; Keiko, Komatsu; Yuya, Asahina; Akiharu, Ueki; Hironobu, Hojo; Yoshiaki, Nakahara; Yukishige, Ito | |||||||
Affiliation |
Department of Applied Biochemistry, Institute of Glycoscience, Tokai University, 4-1-1 Kitakaname, Hiratsuka-shi, Kanagawa 259-1292, Japan. | |||||||
Reference Id |
REF-0000-000372 | |||||||
Source |
J. Org. Chem. 2011, 76, 5229-5239 | |||||||
Doi |
10.1021/jo200149d | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|