
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002830 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002830

|
||||
Regist Date |
2012/06/21 19:54:24 | ||||
| REACTANT | |||||
|
|
|
||||
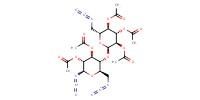
Reactant Type |
azido-saccharide | ||||
Mol |
1.00 mmol | ||||
|
|
|
||||
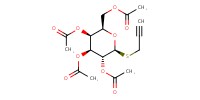
Reactant Type |
alkynyl-thiogalactoside | ||||
Mol |
1.00 mmol per mol of azide | ||||
|
|
|
||||
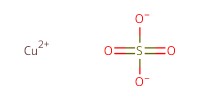
Reactant Type |
CuSO4 | ||||
Mol |
0.25 mmol per mol of azide | ||||
|
|
|
||||
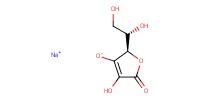
Reactant Type |
sodium ascorbate | ||||
Mol |
0.50 mmol per mol of azide | ||||
| PRODUCT | |||||
MOLECULE ID |
|
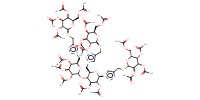
|
|||
Yield |
54% | ||||
| REACTION DETAIL | |||||
Reaction Time |
10 to 16 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
dioxane/H2O = 8/2 (35mL) | ||||
| COMMENT | |||||
| Keywords: biological systems, oligoethyleneglycol chains, trehalose, maltose, maltotriose, direct azidation, microwave irradiation | |||||
| Alternatively, the mixture was stirred under microwave irradiation during 40 minutes. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000369 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102421e | ||||
PubMed ID |
21446743 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (9): 3064-77. | ||||
Article Title |
Synthesis of multivalent glycoclusters from 1-thio-β-D-galactose and their inhibitory activity against the β-galactosidase from E. coli. | ||||
Author |
Alejandro J, Cagnoni; Oscar, Varela; Sébastien G, Gouin; José, Kovensky; María Laura, Uhrig | ||||
Affiliation |
CIHIDECAR-CONICET, Departamento de Qui?mica Orga?nica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina. | ||||
Reference Id |
REF-0000-000370 | ||||
Source |
J. Org. Chem. 2011, 76, 3064-3077 | ||||
Doi |
10.1021/jo102421e | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|