
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002796 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002796

|
||||
Regist Date |
2012/06/21 19:50:10 | ||||
| REACTANT | |||||
|
|
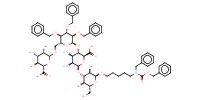
|
||||
Reactant Type |
intermediate 2 | ||||
Weight |
less than 83 mg | ||||
|
|
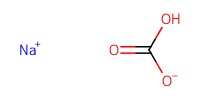
|
||||
Reactant Type |
NaHCO3 (solid) | ||||
Mol |
pH = more than 8 | ||||
|
|
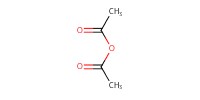
|
||||
Reactant Type |
Ac2O | ||||
Mol |
1.18 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
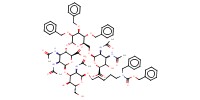
|
|||
Product Type |
intermediate 3 | ||||
Yield |
20%(at least) | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified | ||||
Reaction Temp |
room temp | ||||
Solvent |
THF/H2O = 1/1 (4mL) | ||||
| COMMENT | |||||
| Keywords: mannuronic acids, mannopyranosides, mannopyranosyl methyl uronate, all-cis-linked tetrasaccharide | |||||
| The product was dissolved in THF/H2O (4 mL, 1/1) and treated with 0.45 M aqueous KOH (0.13 mL) to remove any O-acetyls. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000367 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo201179p | ||||
PubMed ID |
21793528 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (18): 7301-15. | ||||
Article Title |
Stereoselective synthesis of 2,3-diamino-2,3-dideoxy-β-D-mannopyranosyl uronates. | ||||
Author |
Marthe T C, Walvoort; Gert-Jan, Moggré; Gerrit, Lodder; Herman S, Overkleeft; Jeroen D C, Codée; Gijsbert A, van der Marel | ||||
Affiliation |
Leiden Institute of Chemistry, Leiden University, P.O. Box 9502, 2300 RA Leiden, The Netherlands. | ||||
Reference Id |
REF-0000-000368 | ||||
Source |
J. Org. Chem. 2011, 76, 7301-7315 | ||||
Doi |
10.1021/jo201179p | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|