
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002787 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002787

|
|||||||
Regist Date |
2012/06/21 19:48:44 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
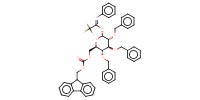
Reactant Type |
donor | |||||||
Mol |
0.65 mmol | |||||||
|
|
|
|||||||
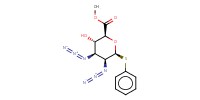
Reactant Type |
acceptor | |||||||
Mol |
0.5 mmol | |||||||
|
|

|
|||||||
Reactant Type |
TfOH | |||||||
Mol |
0.1 mmol | |||||||
| PRODUCT | ||||||||
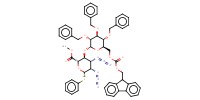
MOLECULE ID |
|
|
||||||
Yield |
96% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified | |||||||
Reaction Temp |
-10 degree C | |||||||
Solvent |
dry Et2O (13mL) | |||||||
Comment |
The reactants were mixed at -40 degree in Celsius before stirred at -10 degree. | |||||||
| COMMENT | ||||||||
| Keywords: mannuronic acids, mannopyranosides, mannopyranosyl methyl uronate, all-cis-linked tetrasaccharide | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000367 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo201179p | |||||||
PubMed ID |
21793528 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (18): 7301-15. | |||||||
Article Title |
Stereoselective synthesis of 2,3-diamino-2,3-dideoxy-β-D-mannopyranosyl uronates. | |||||||
Author |
Marthe T C, Walvoort; Gert-Jan, Moggré; Gerrit, Lodder; Herman S, Overkleeft; Jeroen D C, Codée; Gijsbert A, van der Marel | |||||||
Affiliation |
Leiden Institute of Chemistry, Leiden University, P.O. Box 9502, 2300 RA Leiden, The Netherlands. | |||||||
Reference Id |
REF-0000-000368 | |||||||
Source |
J. Org. Chem. 2011, 76, 7301-7315 | |||||||
Doi |
10.1021/jo201179p | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|