
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002756 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002756

|
|||||||
Regist Date |
2012/06/21 19:45:49 | |||||||
| REACTANT | ||||||||
|
|
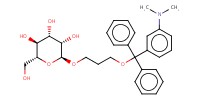
|
|
||||||
Mol |
0.134 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
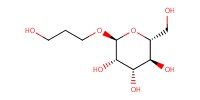
|
|
|||||
Yield |
87% | |||||||
MOLECULE ID |
|
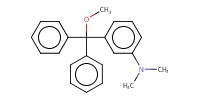
|
||||||
Yield |
43% | |||||||
MOLECULE ID |
|
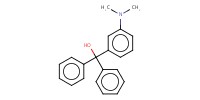
|
||||||
Yield |
5% | |||||||
MOLECULE ID |
|
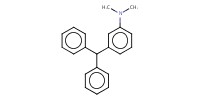
|
||||||
Yield |
11% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
4.5 hours | |||||||
Reaction Temp |
20 to 30 degree C | |||||||
Solvent |
MeOH (26.7mL) | |||||||
Reaction Type |
photochemical cleavage | |||||||
Comment |
irradiation of UV light | |||||||
| The reaction was conducted in a Pyrex test tube kept in a water bath. | ||||||||
| COMMENT | ||||||||
| Keywords: trityl, photolabile, PPGs, electron-donating substituents, m-dimethylamino, photochemical deprotection | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000365 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo200692c | |||||||
PubMed ID |
21612261 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (15): 5873-81. | |||||||
Article Title |
Development of trityl-based photolabile hydroxyl protecting groups. | |||||||
Author |
Lei, Zhou; Haishen, Yang; Pengfei, Wang | |||||||
Affiliation |
Department of Chemistry, University of Alabama at Birmingham, 901 14th Street South, Birmingham, Alabama 35294, USA. | |||||||
Reference Id |
REF-0000-000366 | |||||||
Source |
J. Org. Chem. 2011, 76, 5873-5881 | |||||||
Doi |
10.1021/jo200692c | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|