
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002719 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002719

|
||||
Regist Date |
2012/06/21 19:42:39 | ||||
| REACTANT | |||||
|
|
|
||||
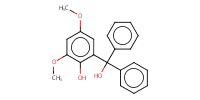
Reactant Type |
2-(hydroxydiphenylmethyl)-4,6-dimethoxyphenol | ||||
Mol |
0.40 mmol | ||||
|
|
|
||||
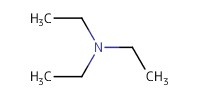
Reactant Type |
TEA | ||||
Mol |
0.44 mmol | ||||
|
|
|
||||
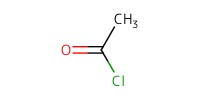
Reactant Type |
AcCl | ||||
Mol |
0.40 mmol | ||||
|
|
|
||||
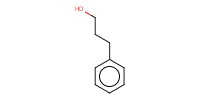
Reactant Type |
3-phenyl-1-propanol | ||||
Mol |
1.60 mmol | ||||
|
|

|
||||
Reactant Type |
NaH | ||||
Mol |
0.48 mmol | ||||
|
|
|
||||
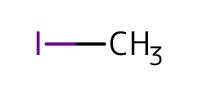
Reactant Type |
MeI | ||||
Mol |
4.00 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
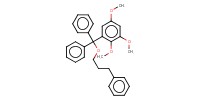
|
|||
Yield |
82% | ||||
| REACTION DETAIL | |||||
Reaction Time |
3 hours, overnight, 3.5 hours | ||||
Reaction Temp |
room temp, room temp, 0 degree C | ||||
Solvent |
CH2Cl2 (0.4mL), CH2Cl2 (0.4mL), THF (0.4mL) | ||||
Comment |
1) 2-(hydroxydiphenylmethyl)-4,6-dimethoxyphenol+TEA, AcCl, 2) +3-phenyl-1-propanol, 3) +all the rest | ||||
| COMMENT | |||||
| Keywords: trityl, photolabile, PPGs, electron-donating substituents, m-dimethylamino, photochemical deprotection | |||||
| The reaction is explained in text only. | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000365 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200692c | ||||
PubMed ID |
21612261 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (15): 5873-81. | ||||
Article Title |
Development of trityl-based photolabile hydroxyl protecting groups. | ||||
Author |
Lei, Zhou; Haishen, Yang; Pengfei, Wang | ||||
Affiliation |
Department of Chemistry, University of Alabama at Birmingham, 901 14th Street South, Birmingham, Alabama 35294, USA. | ||||
Reference Id |
REF-0000-000366 | ||||
Source |
J. Org. Chem. 2011, 76, 5873-5881 | ||||
Doi |
10.1021/jo200692c | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|