
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002674 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002674

|
||||
Regist Date |
2012/06/21 19:37:08 | ||||
| REACTANT | |||||
|
|
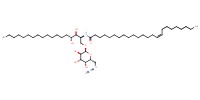
|
||||
Reactant Type |
azide | ||||
Mol |
0.059 mmol | ||||
|
|
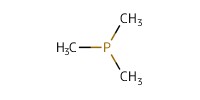
|
||||
Reactant Type |
PMe3 (1.0 M in THF) | ||||
Mol |
0.071 mmol | ||||
|
|

|
||||
Reactant Type |
H2O | ||||
Mol |
0.28 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
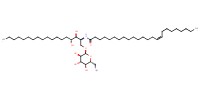
|
|||
Yield |
quantitative | ||||
| REACTION DETAIL | |||||
Reaction Time |
5 hours, 1 hour | ||||
Reaction Temp |
room temp, room temp | ||||
Solvent |
THF (2.5mL), THF (2.5mL) | ||||
Comment |
1) azide+PMe3, 2) +H2O | ||||
| COMMENT | |||||
| Keywords: building block, ceramides, glycolipids, galactosyl-phytosphingosine, KRN7000 | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000361 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102064p | ||||
PubMed ID |
21155575 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (1): 320-3. | ||||
Article Title |
Synthesis of a versatile building block for the preparation of 6-N-derivatized α-galactosyl ceramides: rapid access to biologically active glycolipids. | ||||
Author |
Peter J, Jervis; Liam R, Cox; Gurdyal S, Besra | ||||
Affiliation |
School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom. | ||||
Reference Id |
REF-0000-000362 | ||||
Source |
J. Org. Chem. 2011, 76, 320-323 | ||||
Doi |
10.1021/jo102064p | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|