
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002672 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002672

|
||||
Regist Date |
2012/06/21 19:36:56 | ||||
| REACTANT | |||||
|
|
|
||||
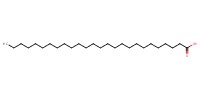
Reactant Type |
hexacosanoic acid | ||||
Mol |
0.11 mmol | ||||
|
|
|
||||
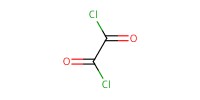
Reactant Type |
(COCl)2 | ||||
Volume |
2.00 mL | ||||
| PRODUCT | |||||
MOLECULE ID |
|
|
|||
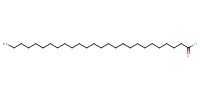
Product Type |
acyl chloride | ||||
Yield |
NOT specified | ||||
| REACTION DETAIL | |||||
Reaction Time |
2 hours | ||||
Reaction Temp |
70 degree C | ||||
Solvent |
(COCl)2 | ||||
| COMMENT | |||||
| Keywords: building block, ceramides, glycolipids, galactosyl-phytosphingosine, KRN7000 | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000361 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102064p | ||||
PubMed ID |
21155575 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (1): 320-3. | ||||
Article Title |
Synthesis of a versatile building block for the preparation of 6-N-derivatized α-galactosyl ceramides: rapid access to biologically active glycolipids. | ||||
Author |
Peter J, Jervis; Liam R, Cox; Gurdyal S, Besra | ||||
Affiliation |
School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom. | ||||
Reference Id |
REF-0000-000362 | ||||
Source |
J. Org. Chem. 2011, 76, 320-323 | ||||
Doi |
10.1021/jo102064p | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|