
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002609 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002609

|
||||
Regist Date |
2012/06/21 19:30:58 | ||||
| REACTANT | |||||
|
|
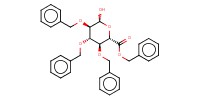
|
||||
Mol |
0.487 mmol | ||||
|
|
|
||||
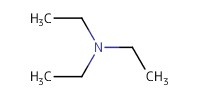
Reactant Type |
TEA | ||||
Mol |
0.84 mmol | ||||
|
|
|
||||
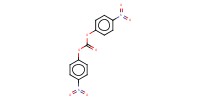
Reactant Type |
(p-NO2C6H4O)2CO (in 3 mL of CH2Cl2) | ||||
Mol |
0.49 mmol | ||||
|
|
|
||||
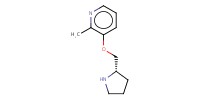
Reactant Type |
(in 4 mL of CH2Cl2) | ||||
Mol |
1 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
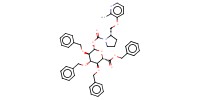
|
|||
Yield |
73% | ||||
| REACTION DETAIL | |||||
Reaction Time |
3 hours, overnight (13 hours) | ||||
Reaction Temp |
ice cooling, room temp | ||||
Solvent |
CH2Cl2 (15+3mL), CH2Cl2 (+4mL) | ||||
Comment |
1) 1b+TEA, (p-NO2C6H4O)2CO 2) +8 | ||||
| The reactants were mixed in the ice cooling solution, and it was gradually allowed to warm to room temperature within the reaction time. (second phase) | |||||
| COMMENT | |||||
| Keywords: carbamyl glucuronidation, metabolism, secondary amine drugs, glucuronyl p-nitrophenyl carbonates | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000357 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200238d | ||||
PubMed ID |
21598979 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (13): 5429-32. | ||||
Article Title |
Reagents for stereoselective preparation of N-carbamyl β-D-glucuronides. | ||||
Author |
William H, Bunnelle | ||||
Affiliation |
Abbott Laboratories, Neuroscience Research, Building AP9A, Department R4MN, 100 Abbott Park Road, Abbott Park, Illinois 60064-6117, United States. william.h.bunnelle@abbott.com | ||||
Reference Id |
REF-0000-000358 | ||||
Source |
J. Org. Chem. 2011, 76, 5429-5432 | ||||
Doi |
10.1021/jo200238d | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|