
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002607 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002607

|
||||
Regist Date |
2012/06/21 19:30:49 | ||||
| REACTANT | |||||
|
|
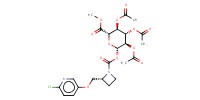
|
||||
Mol |
0.16 mmol | ||||
|
|
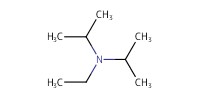
|
||||
Reactant Type |
DIEA | ||||
Volume |
0.5 mL | ||||
| PRODUCT | |||||
MOLECULE ID |
|
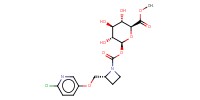
|
|||
Yield |
67% | ||||
| REACTION DETAIL | |||||
Reaction Time |
14 hours, overnight | ||||
Reaction Temp |
0 degree C, room temp | ||||
Solvent |
MeOH/H2O = 5mL/1mL, MeOH/H2O = 5mL/1mL | ||||
Comment |
1) +all, 2) temperature change | ||||
| COMMENT | |||||
| Keywords: carbamyl glucuronidation, metabolism, secondary amine drugs, glucuronyl p-nitrophenyl carbonates | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000357 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200238d | ||||
PubMed ID |
21598979 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (13): 5429-32. | ||||
Article Title |
Reagents for stereoselective preparation of N-carbamyl β-D-glucuronides. | ||||
Author |
William H, Bunnelle | ||||
Affiliation |
Abbott Laboratories, Neuroscience Research, Building AP9A, Department R4MN, 100 Abbott Park Road, Abbott Park, Illinois 60064-6117, United States. william.h.bunnelle@abbott.com | ||||
Reference Id |
REF-0000-000358 | ||||
Source |
J. Org. Chem. 2011, 76, 5429-5432 | ||||
Doi |
10.1021/jo200238d | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|