
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002589 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002589

|
||||
Regist Date |
2012/06/21 19:29:09 | ||||
| REACTANT | |||||
|
|
|
||||
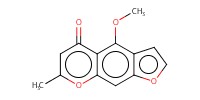
Reactant Type |
visnagin | ||||
|
|

|
||||
Reactant Type |
H2SO4 (25%) | ||||
|
|
|
||||
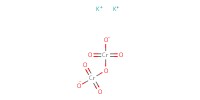
Reactant Type |
K2Cr2O7 (0.5 M) | ||||
|
|

|
||||
Reactant Type |
NaOH (1 M) | ||||
|
|
|
||||
Reactant Type |
H2O2 (30%) | ||||
| PRODUCT | |||||
MOLECULE ID |
|
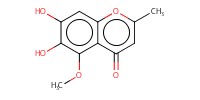
|
|||
Yield |
NOT specified | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified | ||||
Reaction Temp |
NOT specified | ||||
Solvent |
NOT specified | ||||
Comment |
1) visnagin+H2SO4, K2Cr2O7, 2) +NaOH, H2O2 | ||||
| Very few were described regarding this reaction. | |||||
| COMMENT | |||||
| Keywords: 2-methylchromone-7-O-rutinosides, cytotoxicity, human tumor cell lines, acidic labile, 2-methylchromone aglycon | |||||
| The details regarding this reaction are described in another paper in References. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000355 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102325s | ||||
PubMed ID |
21366286 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (7): 2265-8. | ||||
Article Title |
Concise synthesis of 5-methoxy-6-hydroxy-2-methylchromone-7-O- and 5-hydroxy-2-methylchromone-7-O-rutinosides. Investigation of their cytotoxic activities against several human tumor cell lines. | ||||
Author |
Baolin, Wu; Wenpeng, Zhang; Zhonghua, Li; Li, Gu; Xin, Wang; Peng George, Wang | ||||
Affiliation |
Department of Chemistry, The Ohio State University, 484 West 12th Avenue, Columbus, Ohio 43210, USA. | ||||
Reference Id |
REF-0000-000356 | ||||
Source |
J. Org. Chem. 2011, 76, 2265-2268 | ||||
Doi |
10.1021/jo102325s | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|