
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002584 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002584

|
||||
Regist Date |
2012/06/21 19:28:42 | ||||
| REACTANT | |||||
|
|
|
||||
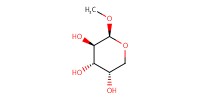
Reactant Type |
carbohydrate substrate | ||||
Mol |
1 equiv. | ||||
|
|
|
||||
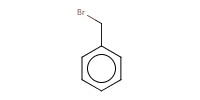
Reactant Type |
R-X (alkyl halide) | ||||
Mol |
1.5 equiv. | ||||
|
|
|
||||
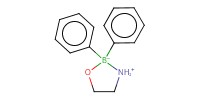
Reactant Type |
2-aminoethyl diphenylborinate | ||||
Mol |
10 mol % | ||||
|
|
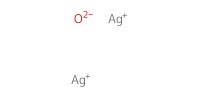
|
||||
Reactant Type |
Ag2O | ||||
Mol |
1.1 equiv. | ||||
| PRODUCT | |||||
MOLECULE ID |
|
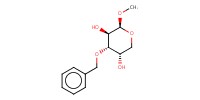
|
|||
Yield |
74% | ||||
| REACTION DETAIL | |||||
Reaction Time |
48 hours | ||||
Reaction Temp |
40 degree C | ||||
Solvent |
MeCN | ||||
| COMMENT | |||||
| Keywords: monoalkylations, regioselective, catalyst, cis-vicinal diol motifs, diphenylborinic ester precatalyst | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000353 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/ol200990e | ||||
PubMed ID |
21591630 | ||||
Journal Name |
Organic letters. (2011) 13 (12): 3090-3. | ||||
Article Title |
Regioselective alkylation of carbohydrate derivatives catalyzed by a diarylborinic acid derivative. | ||||
Author |
Lina, Chan; Mark S, Taylor | ||||
Affiliation |
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto ON M5S 3H6, Canada. | ||||
Reference Id |
REF-0000-000354 | ||||
Source |
Org. Lett., Vol. 13, No. 12, 2011 | ||||
Doi |
10.1021/ol200990e | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|