
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002571 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002571

|
|||||||
Regist Date |
2012/06/21 19:27:39 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
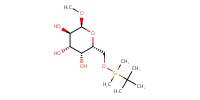
Reactant Type |
carbohydrate substrate | |||||||
Mol |
1 equiv. | |||||||
|
|
|
|||||||
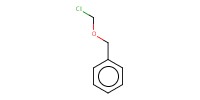
Reactant Type |
R-X (alkyl halide) | |||||||
Mol |
1.5 equiv. | |||||||
|
|
|
|||||||
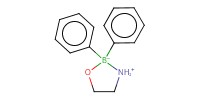
Reactant Type |
2-aminoethyl diphenylborinate | |||||||
Mol |
10 mol % | |||||||
|
|
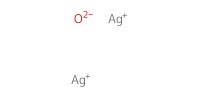
|
|||||||
Reactant Type |
Ag2O | |||||||
Mol |
1.1 equiv. | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
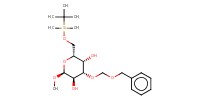
|
|
|||||
Yield |
90% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
48 hours | |||||||
Reaction Temp |
40 degree C | |||||||
Solvent |
MeCN | |||||||
| COMMENT | ||||||||
| Keywords: monoalkylations, regioselective, catalyst, cis-vicinal diol motifs, diphenylborinic ester precatalyst | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000353 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/ol200990e | |||||||
PubMed ID |
21591630 | |||||||
Journal Name |
Organic letters. (2011) 13 (12): 3090-3. | |||||||
Article Title |
Regioselective alkylation of carbohydrate derivatives catalyzed by a diarylborinic acid derivative. | |||||||
Author |
Lina, Chan; Mark S, Taylor | |||||||
Affiliation |
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto ON M5S 3H6, Canada. | |||||||
Reference Id |
REF-0000-000354 | |||||||
Source |
Org. Lett., Vol. 13, No. 12, 2011 | |||||||
Doi |
10.1021/ol200990e | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|