
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002524 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002524

|
|||||||
Regist Date |
2012/06/21 19:23:47 | |||||||
| REACTANT | ||||||||
|
|
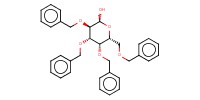
|
|||||||
Reactant Type |
donor | |||||||
Mol |
1 equiv. | |||||||
|
|
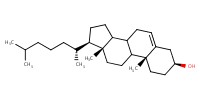
|
|||||||
Reactant Type |
acceptor | |||||||
Mol |
2 equiv. | |||||||
|
|
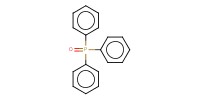
|
|||||||
Reactant Type |
triphenylphosphine oxide | |||||||
Mol |
3.3 equiv. | |||||||
|
|
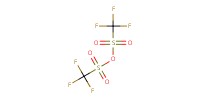
|
|||||||
Reactant Type |
Tf2O | |||||||
Mol |
1.1 equiv. | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
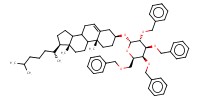
|
|
|||||
Product Type |
alpha | |||||||
Yield |
44%(alpha/beta=63/37) | |||||||
MOLECULE ID |
|
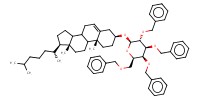
|
|
|||||
Product Type |
beta | |||||||
Yield |
44%(alpha/beta=63/37) | |||||||
MOLECULE ID |
|
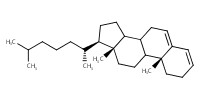
|
||||||
Product Type |
delta3,5-cholestadiene | |||||||
Yield |
NOT specified | |||||||
MOLECULE ID |
|
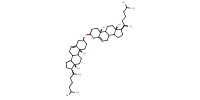
|
||||||
Product Type |
cholesteryl ether | |||||||
Yield |
NOT specified | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified | |||||||
Reaction Temp |
0 degree C | |||||||
Solvent |
CH2Cl2 | |||||||
Comment |
Very few were described regarding this reaction. | |||||||
| COMMENT | ||||||||
| Keywords: the Hendrickson reagent, dehydrative glycosylation, 1-hydroxyglycosyl donors, anomeric oxophosphonium intermediate | ||||||||
| The details regarding this reaction are described in another paper in References. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000351 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo2015856 | |||||||
PubMed ID |
21970416 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (21): 9122-6. | |||||||
Article Title |
Dehydrative glycosylation with the Hendrickson reagent. | |||||||
Author |
Matteo, Mossotti; Luigi, Panza | |||||||
Affiliation |
Dipartimento di Scienze Chimiche, Alimentari, Farmaceutiche e Farmacologiche, Università del Piemonte Orientale, Via Bovio 6, 28100 Novara, Italy. | |||||||
Reference Id |
REF-0000-000352 | |||||||
Source |
J. Org. Chem. 2011, 76, 9122-9126 | |||||||
Doi |
10.1021/jo2015856 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|