
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002503 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002503

|
||||
Regist Date |
2012/06/21 19:20:39 | ||||
| REACTANT | |||||
|
|
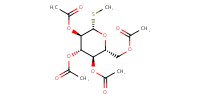
|
||||
Mol |
2.2 mmol | ||||
|
|
|
||||
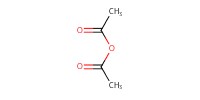
Reactant Type |
Ac2O | ||||
Mol |
1.3 equiv. | ||||
|
|
|
||||
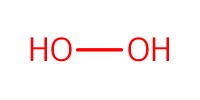
Reactant Type |
H2O2 (34% aqueous solution) | ||||
Mol |
1.2 equiv. | ||||
| PRODUCT | |||||
MOLECULE ID |
|
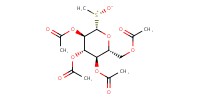
|
|||
Product Type |
Major | ||||
Yield |
71.25% | ||||
MOLECULE ID |
|
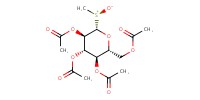
|
|||
Product Type |
Minor | ||||
Yield |
23.75% | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified | ||||
Reaction Temp |
room temp | ||||
Solvent |
CH2Cl2 (5 mL/mmol) | ||||
Comment |
200 mg/mmol of silica gel was included in the solvent. | ||||
| H2O2 was added slowly. | |||||
| COMMENT | |||||
| Keywords: stereochemical properties, alkyl beta-glucosyl sulfoxides, rotamer populations, Taft's steric parameter, NOE experiments | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000347 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo201130x | ||||
PubMed ID |
21830783 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (19): 7769-80. | ||||
Article Title |
Stereochemical properties of glucosyl sulfoxides in solution. | ||||
Author |
Carlos A, Sanhueza; Rosa L, Dorta; Jesús T, Vázquez | ||||
Affiliation |
Instituto Universitario de Bio-Orga?nica Antonio Gonza?lez, Departamento de Qui?mica Orga?nica, Universidad de La Laguna, 38206 La Laguna, Tenerife, Spain. | ||||
Reference Id |
REF-0000-000348 | ||||
Source |
J. Org. Chem. 2011, 76, 7769-7780 | ||||
Doi |
10.1021/jo201130x | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|