
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002488 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002488

|
||||
Regist Date |
2012/06/21 19:19:02 | ||||
| REACTANT | |||||
|
|
|
||||
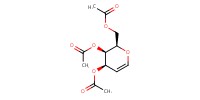
Reactant Type |
Substrate | ||||
Mol |
0.25 mmol | ||||
|
|
|
||||
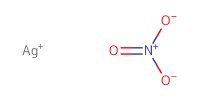
Reactant Type |
AgNO3 | ||||
Mol |
0.25 mmol | ||||
|
|
|
||||
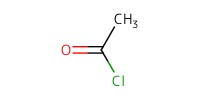
Reactant Type |
AcCl | ||||
Mol |
0.25 mmol | ||||
| PRODUCT | |||||
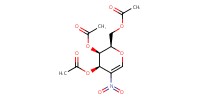
MOLECULE ID |
|
|
|||
Yield |
93% | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour | ||||
Reaction Temp |
55 degree C | ||||
Solvent |
MeCN (3.0 mL) | ||||
Comment |
The addition of AcCl was performed after the addition of all the other reactants. | ||||
| AcCl was added dropwise. | |||||
| The reactants were mixed at 0 degree in Celsius before stirred at 55 degree. | |||||
| COMMENT | |||||
| Keywords: 2-nitroglycals, non-carbohydrate-derived olefins, nitroolefins, acetyl chloride, silver nitrate, acetonitrile | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000345 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200475h | ||||
PubMed ID |
21612270 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (14): 5832-7. | ||||
Article Title |
Acetyl chloride-silver nitrate-acetonitrile: a reagent system for the synthesis of 2-nitroglycals and 2-nitro-1-acetamido sugars from glycals. | ||||
Author |
Pavan K, Kancharla; Y Suman, Reddy; Suresh, Dharuman; Yashwant D, Vankar | ||||
Affiliation |
Department of Chemistry, Indian Institute of Technology, Kanpur, India. | ||||
Reference Id |
REF-0000-000346 | ||||
Source |
J. Org. Chem. 2011, 76, 5832-5837 | ||||
Doi |
10.1021/jo200475h | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|