
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002473 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002473

|
||||
Regist Date |
2012/06/21 19:17:34 | ||||
| REACTANT | |||||
|
|
|
||||
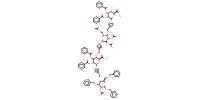
Mol |
1 equiv. | ||||
|
|
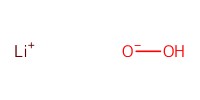
|
||||
Reactant Type |
LiOOH | ||||
Mol |
50 equiv. | ||||
|
|
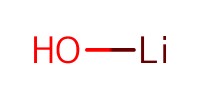
|
||||
Reactant Type |
LiOH (1 M) | ||||
Mol |
25 equiv. | ||||
|
|

|
||||
Reactant Type |
NaOH (2N) | ||||
Volume |
1.0 mL | ||||
|
|
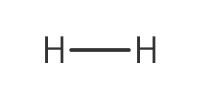
|
||||
Reactant Type |
H2 (gas) | ||||
|
|

|
||||
Reactant Type |
Pd/C (10%) | ||||
Mol |
1 equiv. | ||||
|
|

|
||||
Reactant Type |
HCl (1N) | ||||
Volume |
0.1 mL/5 mg | ||||
| PRODUCT | |||||
MOLECULE ID |
|
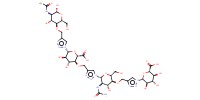
|
|||
Yield |
55% | ||||
| REACTION DETAIL | |||||
Reaction Time |
16 hours, 18 hours, NOT specified | ||||
Reaction Temp |
room temp, room temp, NOT specified | ||||
Solvent |
THF (0.02M), MeOH, MeOH | ||||
Comment |
1) 38+LiOOH, LiOH, 2) +NaOH, 3) +all the rest | ||||
| NaOH was added until the pH of the solution reaches 14. (second phase) | |||||
| COMMENT | |||||
| Keywords: triazole, heparosan, chondroitin, copper catalyzed azide-alkyne cycloaddition, CuAAC, azido-glucuronic acid, glucosamine, galactosamine | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000343 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200076z | ||||
PubMed ID |
21438620 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (9): 3181-93. | ||||
Article Title |
Design and synthesis of unnatural heparosan and chondroitin building blocks. | ||||
Author |
Smritilekha, Bera; Robert J, Linhardt | ||||
Affiliation |
Department of Chemistry and Chemical Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, New York 12180, USA. | ||||
Reference Id |
REF-0000-000344 | ||||
Source |
J. Org. Chem. 2011, 76, 3181-3193 | ||||
Doi |
10.1021/jo200076z | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|