
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002465 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002465

|
|||||||
Regist Date |
2012/06/21 19:16:35 | |||||||
| REACTANT | ||||||||
|
|
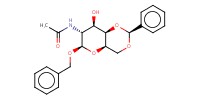
|
|
||||||
Mol |
1.01 mmol | |||||||
|
|
|
|||||||
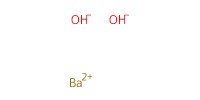
Reactant Type |
Ba(OH)2 | |||||||
Mol |
1.31 mmol | |||||||
|
|
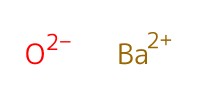
|
|||||||
Reactant Type |
BaO | |||||||
Mol |
3.03 mmol | |||||||
|
|
|
|||||||
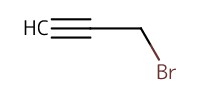
Reactant Type |
propargyl bromide | |||||||
Mol |
1.53 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
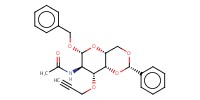
|
|
|||||
Yield |
85% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
10 hours | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
dry DMF (20mL) | |||||||
Comment |
The reactants were mixed at 0 degree in Celsius before stirred at room temperature. | |||||||
| All the reactants except propargyl bromide were mixed before the cooling. | ||||||||
| COMMENT | ||||||||
| Keywords: triazole, heparosan, chondroitin, copper catalyzed azide-alkyne cycloaddition, CuAAC, azido-glucuronic acid, glucosamine, galactosamine | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000343 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo200076z | |||||||
PubMed ID |
21438620 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (9): 3181-93. | |||||||
Article Title |
Design and synthesis of unnatural heparosan and chondroitin building blocks. | |||||||
Author |
Smritilekha, Bera; Robert J, Linhardt | |||||||
Affiliation |
Department of Chemistry and Chemical Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, New York 12180, USA. | |||||||
Reference Id |
REF-0000-000344 | |||||||
Source |
J. Org. Chem. 2011, 76, 3181-3193 | |||||||
Doi |
10.1021/jo200076z | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|