
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002437 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002437

|
|||||||
Regist Date |
2012/06/21 19:14:11 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
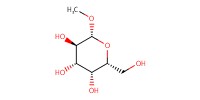
Reactant Type |
carbohydrate compound | |||||||
Mol |
10 mmol | |||||||
|
|
|
|||||||
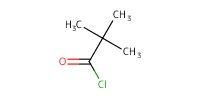
Reactant Type |
pivaloyl chloride | |||||||
Mol |
20 to 50 mmol | |||||||
| PRODUCT | ||||||||
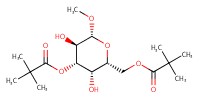
MOLECULE ID |
|
|
|
|||||
Yield |
44% | |||||||
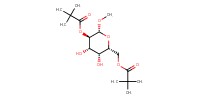
MOLECULE ID |
|
|
|
|||||
Yield |
44% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified | |||||||
Reaction Temp |
0 degree C | |||||||
Solvent |
pyridine | |||||||
Comment |
pivaloyl chloride was added slowly. | |||||||
| COMMENT | ||||||||
| Keywords: acylation, pivaloylation, pivaloyl chloride, esterifying, regioisomers | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000341 | |||||||
Issn |
Electronic | |||||||
PubMed ID |
11672212 | |||||||
Journal Name |
The Journal of organic chemistry. (1998) 63 (17): 6035-6038. | |||||||
Article Title |
Regioselective Acylation of Hexopyranosides with Pivaloyl Chloride. | |||||||
Author |
Lu, Jiang; Tak-Hang, Chan | |||||||
Affiliation |
Department of Chemistry, McGill University, Montréal, Québec H3A 2K6, Canada. | |||||||
Reference Id |
REF-0000-000342 | |||||||
Source |
J. Org. Chem. 1998, 63, 6035-6038 | |||||||
Doi |
10.1021/jo980294v | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|