
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002416 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002416

|
|||||||
Regist Date |
2012/06/21 19:12:21 | |||||||
| REACTANT | ||||||||
|
|
|
|||||||
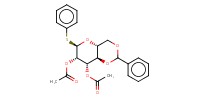
Reactant Type |
thiomannopyranoside | |||||||
Mol |
0.3 mmol | |||||||
|
|
|
|
||||||
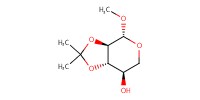
Reactant Type |
xylopyranoside | |||||||
Mol |
0.33 mmol | |||||||
|
|
|
|||||||
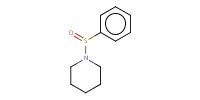
Reactant Type |
BSP | |||||||
Mol |
0.36 mmol | |||||||
|
|
|
|||||||
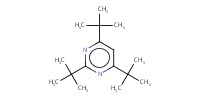
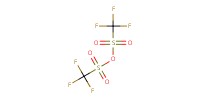
Reactant Type |
TTBP | |||||||
Mol |
0.45 mmol | |||||||
|
|
|
|||||||
Reactant Type |
Tf2O | |||||||
Mol |
0.36 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
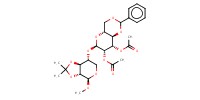
|
||||||
Yield |
84% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
30 minutes, 2.5 hours | |||||||
Reaction Temp |
-65 to -60 degree C, -60 degree C | |||||||
Solvent |
distilled CH2Cl2, distilled CH2Cl2 | |||||||
Comment |
1) +all except xylopyranoside, 2) +xylopyranoside | |||||||
| Before the reaction, thiomannopyranoside, BSP, TTBP, and MS 4A were mixed and stirred for 30 minutes at room temperature and then for 5 minutes at -65 degree in Celsius. | ||||||||
| MS 4A was included in the solvent. | ||||||||
| Both Tf2O and xylopyranoside were added dropwise. | ||||||||
| COMMENT | ||||||||
| Keywords: xylomannan, antifreeze, Alaskan beetle, Upis ceramboides | ||||||||
| There are multiple phases in this reaction. | ||||||||
| ATTENTION: There are typos in the written method. (for compound 25 and 26, protocols and xylopyranosides are assigned inversely) | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000339 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo201780e | |||||||
PubMed ID |
21955117 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (21): 8611-20. | |||||||
Article Title |
Synthesis and structural verification of the xylomannan antifreeze substance from the freeze-tolerant Alaskan beetle Upis ceramboides. | |||||||
Author |
David, Crich; Md Yeajur, Rahaman | |||||||
Affiliation |
Department of Chemistry, Wayne State University, 5101 Cass Avenue, Detroit, Michigan 48202, United States. dcrich@chem.wayne.edu | |||||||
Reference Id |
REF-0000-000340 | |||||||
Source |
J. Org. Chem. 2011, 76, 8611-8620 | |||||||
Doi |
10.1021/jo201780e | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|