
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002403 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002403

|
||||
Regist Date |
2012/06/21 19:10:11 | ||||
| REACTANT | |||||
|
|
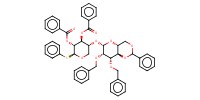
|
||||
Reactant Type |
thioglycoside | ||||
Mol |
100 micro mole | ||||
|
|
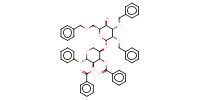
|
||||
Reactant Type |
acceptor (in CH2Cl2) | ||||
Mol |
50 micro mole | ||||
|
|
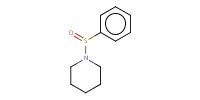
|
||||
Reactant Type |
BSP | ||||
Mol |
120 micro mole | ||||
|
|
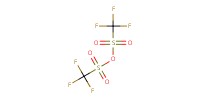
|
||||
Reactant Type |
Tf2O | ||||
Mol |
120 micro mole | ||||
|
|
|
||||
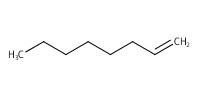
Reactant Type |
1-octene | ||||
Mol |
1 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
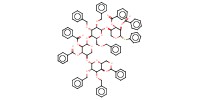
|
|||
Yield |
79% | ||||
| REACTION DETAIL | |||||
Reaction Time |
30 minutes, 5 + 5 minutes, 2.5 hours | ||||
Reaction Temp |
-65 to -60 degree C, -78 degree C, -60 degree C | ||||
Solvent |
distilled CH2Cl2, distilled CH2Cl2, distilled CH2Cl2 | ||||
Comment |
1) +all except 1-octene and acceptor, 2) +1-octene, 3) +acceptor | ||||
| Before the reaction, thioglycoside, BSP, and MS 4A were mixed and stirred for 0.5 hour at room temperature and then for 5 minutes at -65 degree in Celsius. | |||||
| MS 4A was included in the solvent. | |||||
| 1-octene was added 5 minutes after the initiation of the reaction. (second phase) | |||||
| acceptor was added dropwise. | |||||
| COMMENT | |||||
| Keywords: xylomannan, antifreeze, Alaskan beetle, Upis ceramboides | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000339 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo201780e | ||||
PubMed ID |
21955117 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (21): 8611-20. | ||||
Article Title |
Synthesis and structural verification of the xylomannan antifreeze substance from the freeze-tolerant Alaskan beetle Upis ceramboides. | ||||
Author |
David, Crich; Md Yeajur, Rahaman | ||||
Affiliation |
Department of Chemistry, Wayne State University, 5101 Cass Avenue, Detroit, Michigan 48202, United States. dcrich@chem.wayne.edu | ||||
Reference Id |
REF-0000-000340 | ||||
Source |
J. Org. Chem. 2011, 76, 8611-8620 | ||||
Doi |
10.1021/jo201780e | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|