
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002395 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002395

|
||||
Regist Date |
2012/06/21 19:09:15 | ||||
| REACTANT | |||||
|
|
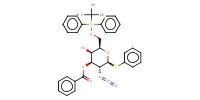
|
||||
Mol |
0.256 mmol | ||||
|
|
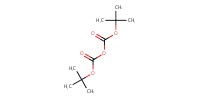
|
||||
Reactant Type |
Boc2O | ||||
Mol |
0.76 mmol | ||||
|
|
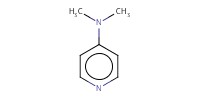
|
||||
Reactant Type |
DMAP | ||||
Mol |
0.076 mmol | ||||
|
|
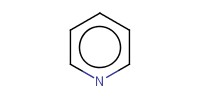
|
||||
Reactant Type |
pyridine | ||||
Mol |
0.76 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
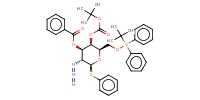
|
|||
Yield |
90% | ||||
| REACTION DETAIL | |||||
Reaction Time |
15 minutes | ||||
Reaction Temp |
room temp | ||||
Solvent |
CH2Cl2 | ||||
Comment |
The addition of Boc2O was performed before the addition of DMAP. | ||||
| The addition of pyridine was performed after the addition of all the other reactants. | |||||
| COMMENT | |||||
| Keywords: mannose, glucosamine, galactosamine, thioglycosides, 3-O-acylation, 4,6-O-benzylidenation, azide displacement, Lattrell-Dax inversion | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000337 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200342v | ||||
PubMed ID |
21510706 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (11): 4703-9. | ||||
Article Title |
Rapid transformation of D-mannose into orthogonally protected D-glucosamine and D-galactosamine thioglycosides. | ||||
Author |
Madhu, Emmadi; Suvarn S, Kulkarni | ||||
Affiliation |
Department of Chemistry, Indian Institute of Technology Bombay, Mumbai, India. | ||||
Reference Id |
REF-0000-000338 | ||||
Source |
J. Org. Chem. 2011, 76, 4703-4709 | ||||
Doi |
10.1021/jo200342v | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|