
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002389 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002389

|
||||
Regist Date |
2012/06/21 19:08:40 | ||||
| REACTANT | |||||
|
|
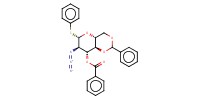
|
||||
Reactant Type |
sugar | ||||
Mol |
0.24 mmol | ||||
|
|
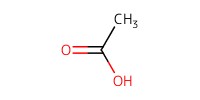
|
||||
Reactant Type |
AcOH (80% in H2O, solvent) | ||||
Volume |
5 mL | ||||
|
|
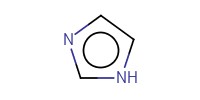
|
||||
Reactant Type |
imidazole | ||||
Mol |
0.51 mmol | ||||
|
|
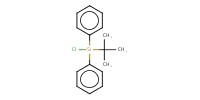
|
||||
Reactant Type |
TBDPSCl | ||||
Mol |
0.245 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
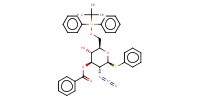
|
|||
Yield |
81% | ||||
| REACTION DETAIL | |||||
Reaction Time |
90 minutes, 15 minutes | ||||
Reaction Temp |
90 degree C (reflux), room temp | ||||
Solvent |
80% AcOH, CH3CN | ||||
Comment |
1) sugar+AcOH, 2) +all the rest | ||||
| COMMENT | |||||
| Keywords: mannose, glucosamine, galactosamine, thioglycosides, 3-O-acylation, 4,6-O-benzylidenation, azide displacement, Lattrell-Dax inversion | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000337 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200342v | ||||
PubMed ID |
21510706 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (11): 4703-9. | ||||
Article Title |
Rapid transformation of D-mannose into orthogonally protected D-glucosamine and D-galactosamine thioglycosides. | ||||
Author |
Madhu, Emmadi; Suvarn S, Kulkarni | ||||
Affiliation |
Department of Chemistry, Indian Institute of Technology Bombay, Mumbai, India. | ||||
Reference Id |
REF-0000-000338 | ||||
Source |
J. Org. Chem. 2011, 76, 4703-4709 | ||||
Doi |
10.1021/jo200342v | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|