
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002379 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002379

|
||||
Regist Date |
2012/06/21 19:07:48 | ||||
| REACTANT | |||||
|
|
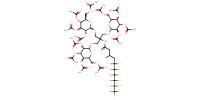
|
||||
Mol |
0.37 mmol | ||||
|
|
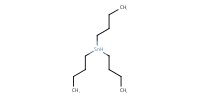
|
||||
Reactant Type |
Bu3SnH | ||||
Mol |
0.44 mmol | ||||
|
|
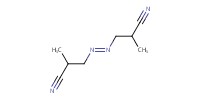
|
||||
Reactant Type |
AIBN | ||||
Mol |
0.03 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
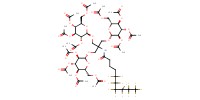
|
|||
Yield |
69% | ||||
| REACTION DETAIL | |||||
Reaction Time |
24 hours | ||||
Reaction Temp |
66 degree C | ||||
Solvent |
anhydrous THF | ||||
Comment |
The reaction was conducted in a sealed tube. | ||||
| COMMENT | |||||
| Keywords: hemifluorinated surfactants, membrane proteins, perfluorohexane-based, AIBN, adsorption, micellization | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000335 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102245c | ||||
PubMed ID |
21384802 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (7): 2084-93. | ||||
Article Title |
Propyl-ended hemifluorinated surfactants: synthesis and self-assembling properties. | ||||
Author |
Maher, Abla; Grégory, Durand; Bernard, Pucci | ||||
Affiliation |
Université d'Avignon et des Pays de Vaucluse, Faculté des Sciences, Equipe Chimie Bioorganique et Systèmes Amphiphiles, 33 rue Louis Pasteur, F-84000 Avignon, France. | ||||
Reference Id |
REF-0000-000336 | ||||
Source |
J. Org. Chem. 2011, 76, 2084-2093 | ||||
Doi |
10.1021/jo102245c | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|