
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002351 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002351

|
||||
Regist Date |
2012/06/21 19:04:42 | ||||
| REACTANT | |||||
|
|
|
||||
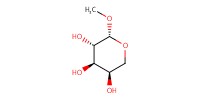
Reactant Type |
acceptor | ||||
Mol |
1.1 equiv. | ||||
|
|
|
||||
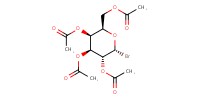
Reactant Type |
donor | ||||
Mol |
1 equiv. | ||||
|
|
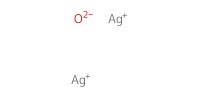
|
||||
Reactant Type |
Ag2O | ||||
Mol |
1 equiv. | ||||
|
|
|
||||
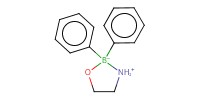
Reactant Type |
catalyst | ||||
Mol |
10 mol% | ||||
| PRODUCT | |||||
MOLECULE ID |
|
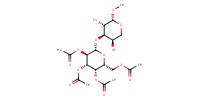
|
|||
Yield |
78% | ||||
| REACTION DETAIL | |||||
Reaction Time |
16 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
dry MeCN | ||||
| COMMENT | |||||
| Keywords: diphenylborinic acid, Koenigs-Knorr glycosylations, cis-1,2-diol, glycosyl halide donors, glycosidic linkages | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000333 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/ja2062715 | ||||
PubMed ID |
21838223 | ||||
Journal Name |
Journal of the American Chemical Society. (2011) 133 (35): 13926-9. | ||||
Article Title |
Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. | ||||
Author |
Christina, Gouliaras; Doris, Lee; Lina, Chan; Mark S, Taylor | ||||
Affiliation |
Department of Chemistry, University of Toronto, Ontario, Canada. | ||||
Reference Id |
REF-0000-000334 | ||||
Source |
J. Am. Chem. Soc. 2011, 133, 13926-13929 | ||||
Doi |
10.1021/ja2062715 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|