
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002338 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002338

|
|||||||
Regist Date |
2012/06/21 19:03:37 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
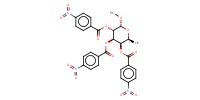
Reactant Type |
tri-O-para-nitrobenzoyl | |||||||
Mol |
0.80 mmol | |||||||
|
|

|
|||||||
Reactant Type |
HBr (33% in AcOH) | |||||||
Volume |
1.9 mL | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
|
||||||
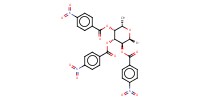
Product Type |
tri-O-para-nitrobenzoyl bromide | |||||||
Yield |
14% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
24 hours | |||||||
Reaction Temp |
35 degree C | |||||||
Solvent |
CH2Cl2 | |||||||
Comment |
Preparation of Glycosyl Donors | |||||||
| COMMENT | ||||||||
| Keywords: diphenylborinic acid, Koenigs-Knorr glycosylations, cis-1,2-diol, glycosyl halide donors, glycosidic linkages | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000333 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/ja2062715 | |||||||
PubMed ID |
21838223 | |||||||
Journal Name |
Journal of the American Chemical Society. (2011) 133 (35): 13926-9. | |||||||
Article Title |
Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. | |||||||
Author |
Christina, Gouliaras; Doris, Lee; Lina, Chan; Mark S, Taylor | |||||||
Affiliation |
Department of Chemistry, University of Toronto, Ontario, Canada. | |||||||
Reference Id |
REF-0000-000334 | |||||||
Source |
J. Am. Chem. Soc. 2011, 133, 13926-13929 | |||||||
Doi |
10.1021/ja2062715 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|