
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002316 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002316

|
||||
Regist Date |
2012/06/21 19:01:44 | ||||
| REACTANT | |||||
|
|
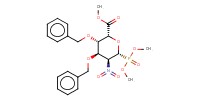
|
||||
Mol |
0.39 mmol | ||||
|
|

|
||||
Reactant Type |
Raney nickel (platinized) | ||||
Weight |
1.5 g | ||||
|
|
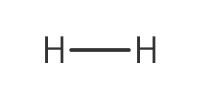
|
||||
Reactant Type |
H2 (gas, 45 psi) | ||||
|
|
|
||||
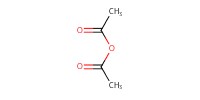
Reactant Type |
Ac2O (solvent) | ||||
Volume |
2 mL | ||||
| PRODUCT | |||||
MOLECULE ID |
|
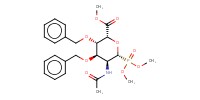
|
|||
Yield |
68% | ||||
| REACTION DETAIL | |||||
Reaction Time |
24 hours, overnight | ||||
Reaction Temp |
room temp, room temp | ||||
Solvent |
EtOH, Ac2O | ||||
Comment |
1) +all except Ac2O, 2) +Ac2O | ||||
| COMMENT | |||||
| Keywords: 2-acylamino uronic acid, glycosyl phosphonates, oxidation, uronate 2-nitro-glycal, thermodynamic 1,2-trans-di-equatorial phosphonylation | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000331 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo2002193 | ||||
PubMed ID |
21495699 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (10): 4099-104. | ||||
Article Title |
Accessing C-1 phosphonylated 2-acylamino uronic acids via 2-nitro-glycals. | ||||
Author |
Beenu, Bhatt; Robin J, Thomson; Mark, von Itzstein | ||||
Affiliation |
Institute for Glycomics, Griffith University, Gold Coast Campus, Queensland 4222, Australia. | ||||
Reference Id |
REF-0000-000332 | ||||
Source |
J. Org. Chem. 2011, 76, 4099-4104 | ||||
Doi |
10.1021/jo2002193 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|