
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002305 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002305

|
||||
Regist Date |
2012/06/21 19:00:23 | ||||
| REACTANT | |||||
|
|
|
||||
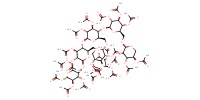
Reactant Type |
thioacetate | ||||
Mol |
1.0 equiv. | ||||
|
|
|
||||
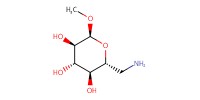
Reactant Type |
amino sugar (in MeOH) | ||||
Mol |
5.0 equiv. | ||||
|
|
|
||||
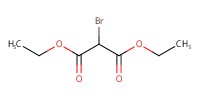
Reactant Type |
BrCH(CO2Et)2 | ||||
Mol |
2.5 equiv. | ||||
| PRODUCT | |||||
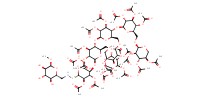
MOLECULE ID |
|
|
|||
Yield |
60% | ||||
| REACTION DETAIL | |||||
Reaction Time |
overnight | ||||
Reaction Temp |
40 degree C | ||||
Solvent |
anhydrous MeOH | ||||
Comment |
The thioacetate and BrCH(CO2Et)2 were mixed and stirred for 30 minutes at room temperature before the reaction. | ||||
| COMMENT | |||||
| Keywords: sulfonamide-bridged, glycosyl thioacetates, amino sugar substates | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000329 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo2001269 | ||||
PubMed ID |
21401206 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (9): 2965-75. | ||||
Article Title |
Synthesis of sulfonamide-bridged glycomimetics. | ||||
Author |
Marie, Lopez; Laurent F, Bornaghi; Hugues, Driguez; Sally-Ann, Poulsen | ||||
Affiliation |
Eskitis Institute, Griffith University, Nathan Campus, Queensland, 4111, Australia. | ||||
Reference Id |
REF-0000-000330 | ||||
Source |
J. Org. Chem. 2011, 76, 2965-2975 | ||||
Doi |
10.1021/jo2001269 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|