
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002285 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002285

|
||||
Regist Date |
2012/06/21 18:57:51 | ||||
| REACTANT | |||||
|
|
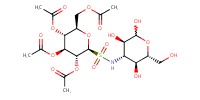
|
||||
Reactant Type |
per-O-acetylated | ||||
Mol |
1.0 equiv. | ||||
|
|
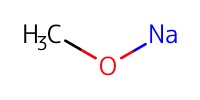
|
||||
Reactant Type |
NaOMe (0.05 M in MeOH) | ||||
| PRODUCT | |||||
MOLECULE ID |
|
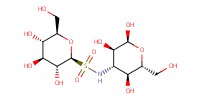
|
|||
Product Type |
alpha | ||||
Yield |
84%(alpha/beta=40/60) | ||||
MOLECULE ID |
|
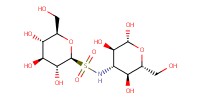
|
|||
Product Type |
beta | ||||
Yield |
84%(alpha/beta=40/60) | ||||
| REACTION DETAIL | |||||
Reaction Time |
30 minutes to overnight | ||||
Reaction Temp |
room temp | ||||
Solvent |
MeOH | ||||
Comment |
The reactants were mixed at 0 degree in Celsius before stirred at room temperature. | ||||
| The pH of the solution was kept at 12 by adjusting the amount of NaOMe. | |||||
| COMMENT | |||||
| Keywords: sulfonamide-bridged, glycosyl thioacetates, amino sugar substates | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000329 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo2001269 | ||||
PubMed ID |
21401206 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (9): 2965-75. | ||||
Article Title |
Synthesis of sulfonamide-bridged glycomimetics. | ||||
Author |
Marie, Lopez; Laurent F, Bornaghi; Hugues, Driguez; Sally-Ann, Poulsen | ||||
Affiliation |
Eskitis Institute, Griffith University, Nathan Campus, Queensland, 4111, Australia. | ||||
Reference Id |
REF-0000-000330 | ||||
Source |
J. Org. Chem. 2011, 76, 2965-2975 | ||||
Doi |
10.1021/jo2001269 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|