
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002264 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002264

|
|||||||
Regist Date |
2012/06/21 18:55:17 | |||||||
| REACTANT | ||||||||
|
|
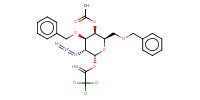
|
|||||||
Reactant Type |
donor | |||||||
Mol |
1.2 equiv. | |||||||
|
|
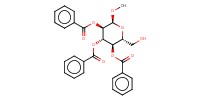
|
|
||||||
Reactant Type |
acceptor | |||||||
Mol |
1.0 equiv. | |||||||
|
|
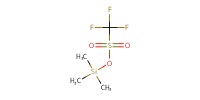
|
|||||||
Reactant Type |
TMSOTf | |||||||
Mol |
0.15 equiv. | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
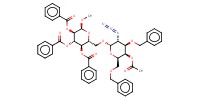
|
||||||
Product Type |
alpha | |||||||
Yield |
53%(alpha/beta=6/1) | |||||||
MOLECULE ID |
|
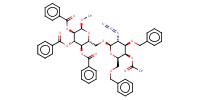
|
||||||
Product Type |
beta | |||||||
Yield |
53%(alpha/beta=6/1) | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
anhydrous CH2Cl2 | |||||||
Comment |
Activated molecular sieves (50 mg/mL solvent) were included in the solvent. | |||||||
| The donor, the acceptor, and activated molecular sieves were mixed and stirred for 0.5 hour at room temperature before the reaction. | ||||||||
| COMMENT | ||||||||
| Keywords: stereoselectivity, 2-azido-2-deoxygalactosyl donors, acetyl groups, temperature, computational methods | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000327 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo1025157 | |||||||
PubMed ID |
21574599 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (13): 5207-18. | |||||||
Article Title |
Study of the stereoselectivity of 2-azido-2-deoxygalactosyl donors: remote protecting group effects and temperature dependency. | |||||||
Author |
Jane, Kalikanda; Zhitao, Li | |||||||
Affiliation |
Department of Chemistry, Binghamton University, Binghamton, New York 13902, United States. | |||||||
Reference Id |
REF-0000-000328 | |||||||
Source |
J. Org. Chem. 2011, 76, 5207-5218 | |||||||
Doi |
10.1021/jo1025157 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|