
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002187 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002187

|
||||
Regist Date |
2012/06/21 18:46:26 | ||||
| REACTANT | |||||
|
|
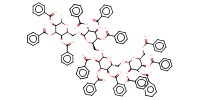
|
||||
Mol |
0.11 mmol | ||||
|
|
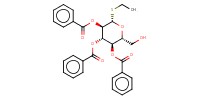
|
||||
Mol |
0.10 mmol | ||||
|
|
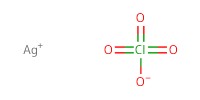
|
||||
Reactant Type |
AgClO4 | ||||
Mol |
0.22 mmol | ||||
|
|
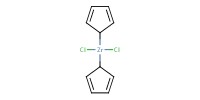
|
||||
Reactant Type |
Cp2ZrCl2 | ||||
Mol |
0.22 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
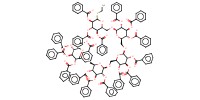
|
|||
Yield |
84% | ||||
| REACTION DETAIL | |||||
Reaction Time |
16 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
(ClCH2)2 | ||||
Comment |
The donor, the acceptor, and molecular sieves (4 A) were mixed and stirred for 1 hour at room temperature before the reaction. | ||||
| MS 4A was included in the solvent. | |||||
| COMMENT | |||||
| Keywords: S-thiazolinyl, STaz, S-benzoxazolyl, SBox, S-ethyl, anomeric leaving groups, activation, monosaccharide building blocks | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000325 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo201117s | ||||
PubMed ID |
21797272 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (18): 7388-98. | ||||
Article Title |
On orthogonal and selective activation of glycosyl thioimidates and thioglycosides: application to oligosaccharide assembly. | ||||
Author |
Sophon, Kaeothip; Alexei V, Demchenko | ||||
Affiliation |
Department of Chemistry and Biochemistry, University of Missouri-St. Louis, One University Boulevard, St. Louis, Missouri 63121, USA. | ||||
Reference Id |
REF-0000-000326 | ||||
Source |
J. Org. Chem. 2011, 76, 7388-7398 | ||||
Doi |
10.1021/jo201117s | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|