
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002169 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002169

|
||||
Regist Date |
2012/06/21 18:44:27 | ||||
| REACTANT | |||||
|
|
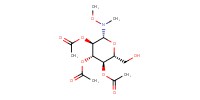
|
||||
Mol |
0.28 mmol | ||||
|
|
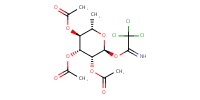
|
||||
Reactant Type |
trichloroacetimidate | ||||
Mol |
0.28 mmol | ||||
|
|
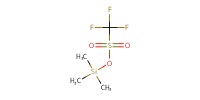
|
||||
Reactant Type |
TMSOTf | ||||
Volume |
10 micro L | ||||
| PRODUCT | |||||
MOLECULE ID |
|
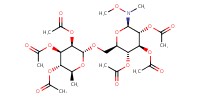
|
|||
Yield |
91% | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour | ||||
Reaction Temp |
room temp | ||||
Solvent |
dry CH2Cl2 | ||||
Comment |
MS 4A was included in the solvent. | ||||
| The donor, the acceptor, and MS 4A were mixed and stirred for 30 minutes before the reaction. | |||||
| COMMENT | |||||
| Keywords: N,O-dimethyloxyamine-N-glycosides, protecting group manipulations, N-chlorosuccinimide, NCS, thioglycoside, trichloroacetimidate, complex oligosaccharide synthesis | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000323 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102372m | ||||
PubMed ID |
21332162 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (6): 1918-21. | ||||
Article Title |
Use of N,O-dimethylhydroxylamine as an anomeric protecting group in carbohydrate synthesis. | ||||
Author |
Somnath, Dasgupta; Mark, Nitz | ||||
Affiliation |
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, ON, Canada M5S 3H6. | ||||
Reference Id |
REF-0000-000324 | ||||
Source |
J. Org. Chem. 2011, 76, 1918-1921 | ||||
Doi |
10.1021/jo102372m | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|