
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002136 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002136

|
|||||||
Regist Date |
2012/06/21 18:37:15 | |||||||
| REACTANT | ||||||||
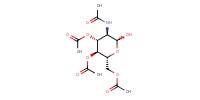
|
|
|
|||||||
|
|
|
|||||||
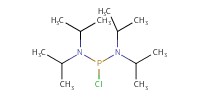
Reactant Type |
(i-Pr2N)2PCl | |||||||
|
|
|
|||||||
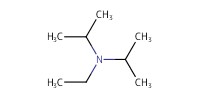
Reactant Type |
DIPEA | |||||||
| PRODUCT | ||||||||
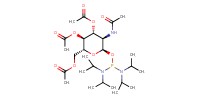
MOLECULE ID |
|
|
|
|||||
Yield |
32% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
CH2Cl2 | |||||||
Comment |
MS 4A was included in the solvent. | |||||||
| Very few were described regarding this reaction. | ||||||||
| COMMENT | ||||||||
| Keywords: mannose-6-phosphate, M6P, N-linked glycans, eukaryotic cells, N-acetylglucosamine, GlcNAc, sequential deprotection | ||||||||
| The procedure regarding this reaction is discussed in another paper in References. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000321 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo2010999 | |||||||
PubMed ID |
21955083 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (21): 8682-9. | |||||||
Article Title |
Chemical synthesis of N-linked glycans carrying both mannose-6-phosphate and GlcNAc-mannose-6-phosphate motifs. | |||||||
Author |
Yunpeng, Liu; Gong, Chen | |||||||
Affiliation |
Department of Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, United States. | |||||||
Reference Id |
REF-0000-000322 | |||||||
Source |
J. Org. Chem., 2011, 76 (21), pp 8682-8689 | |||||||
Doi |
10.1021/jo2010999 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|