
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002122 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002122

|
|||||||
Regist Date |
2012/06/21 18:36:24 | |||||||
| REACTANT | ||||||||
|
|
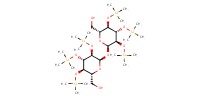
|
|
||||||
Reactant Type |
(in 1 mL of CH2Cl2) | |||||||
Mol |
0.14 mmol | |||||||
|
|
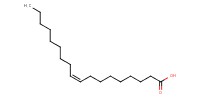
|
|||||||
Reactant Type |
oleic acid | |||||||
Mol |
0.14 mmol | |||||||
|
|
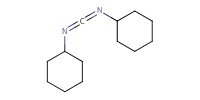
|
|||||||
Reactant Type |
DCC | |||||||
Mol |
0.28 mmol | |||||||
|
|
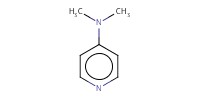
|
|||||||
Reactant Type |
DMAP | |||||||
Mol |
0.07 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
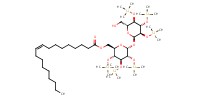
|
|
|||||
Yield |
60% | |||||||
MOLECULE ID |
|
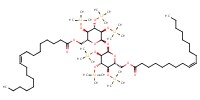
|
|
|||||
Yield |
17% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
more than 5 hours | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
CH2Cl2 | |||||||
Comment |
The reactants were mixed at 0 degree in Celsius, and allowed to warm to room temperature. | |||||||
| The addition of 4 was performed after the addition of all the other reactants. | ||||||||
| 4 was added in a slow, dropwise manner. | ||||||||
| COMMENT | ||||||||
| Keywords: maradolipid, C. elegans, trehalose | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000319 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo200979n | |||||||
PubMed ID |
21739985 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (16): 6866-70. | |||||||
Article Title |
Synthesis of maradolipid. | |||||||
Author |
Vikram A, Sarpe; Suvarn S, Kulkarni | |||||||
Affiliation |
Department of Chemistry, Indian Institute of Technology Bombay, Mumbai 400076, India. | |||||||
Reference Id |
REF-0000-000320 | |||||||
Source |
J. Org. Chem. 2011, 76, 6866-6870 | |||||||
Doi |
10.1021/jo200979n | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|