
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002099 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002099

|
|||||||
Regist Date |
2012/06/21 18:34:26 | |||||||
| REACTANT | ||||||||
|
|
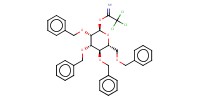
|
|
||||||
Mol |
0.14 mmol | |||||||
|
|
|
|||||||
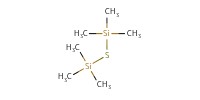
Reactant Type |
(Me3Si)2S | |||||||
Mol |
0.14 mmol | |||||||
|
|
|
|||||||
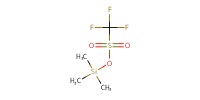
Reactant Type |
TMSOTf | |||||||
Mol |
0.02 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
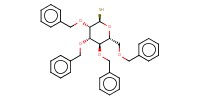
|
||||||
Product Type |
alpha | |||||||
Yield |
62%(alpha/beta=1/1) | |||||||
MOLECULE ID |
|
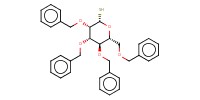
|
||||||
Product Type |
beta | |||||||
Yield |
62%(alpha/beta=1/1) | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
30 minutes | |||||||
Reaction Temp |
0 degree C | |||||||
Solvent |
CH2Cl2 | |||||||
| COMMENT | ||||||||
| Keywords: glycosylthiols, bis-trimethyl-silyl sulfide, sterically hindered S-nucleophile | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000317 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo200624e | |||||||
PubMed ID |
21800823 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (18): 7539-45. | |||||||
Article Title |
Synthesis of glycosylthiols and reactivity studies. | |||||||
Author |
Ravindra T, Dere; Amit, Kumar; Vipin, Kumar; Xiangming, Zhu; Richard R, Schmidt | |||||||
Affiliation |
Fachbereich Chemie, Universita?t Konstanz, Fach 725, D-78457 Konstanz, Germany. | |||||||
Reference Id |
REF-0000-000318 | |||||||
Source |
J. Org. Chem. 2011, 76, 7539-7545 | |||||||
Doi |
10.1021/jo200624e | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|