
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002086 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002086

|
||||
Regist Date |
2012/06/21 18:33:01 | ||||
| REACTANT | |||||
|
|
|
||||
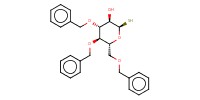
Mol |
0.10 mmol | ||||
|
|
|
||||
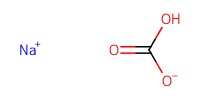
Reactant Type |
NaHCO3 (pH 8.5 solution) | ||||
|
|
|
||||
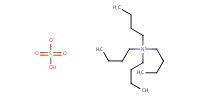
Reactant Type |
TBAHS | ||||
|
|
|
||||
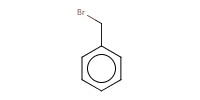
Reactant Type |
BnBr | ||||
| PRODUCT | |||||
MOLECULE ID |
|
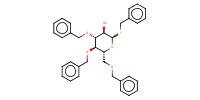
|
|||
Yield |
67% | ||||
| REACTION DETAIL | |||||
Reaction Time |
12 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
EtOAc | ||||
Comment |
The procedure is considered to be the same as the synthesis of compound 3. | ||||
| COMMENT | |||||
| Keywords: glycosylthiols, bis-trimethyl-silyl sulfide, sterically hindered S-nucleophile | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000317 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo200624e | ||||
PubMed ID |
21800823 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (18): 7539-45. | ||||
Article Title |
Synthesis of glycosylthiols and reactivity studies. | ||||
Author |
Ravindra T, Dere; Amit, Kumar; Vipin, Kumar; Xiangming, Zhu; Richard R, Schmidt | ||||
Affiliation |
Fachbereich Chemie, Universita?t Konstanz, Fach 725, D-78457 Konstanz, Germany. | ||||
Reference Id |
REF-0000-000318 | ||||
Source |
J. Org. Chem. 2011, 76, 7539-7545 | ||||
Doi |
10.1021/jo200624e | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|