
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002073 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-002073

|
|||||||
Regist Date |
2012/06/21 18:31:41 | |||||||
| REACTANT | ||||||||
|
|
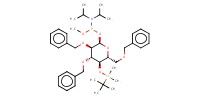
|
|
||||||
Mol |
0.45 mmol | |||||||
|
|
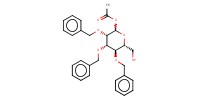
|
|||||||
Mol |
0.37 mmol | |||||||
|
|
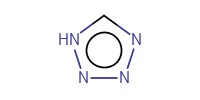
|
|||||||
Reactant Type |
1H-tetrazole | |||||||
Mol |
0.90 mmol | |||||||
|
|
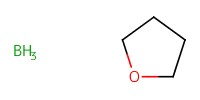
|
|||||||
Reactant Type |
BH3*THF (1 M in THF) | |||||||
Mol |
1.87 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
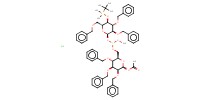
|
||||||
Yield |
84% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
25 minutes, 15 minutes | |||||||
Reaction Temp |
room temp, room temp | |||||||
Solvent |
dry MeCN, dry THF | |||||||
Comment |
1) +all except BH3*THF, 2) +BH3*THF | |||||||
| COMMENT | ||||||||
| Keywords: alpha-D-glycosyl boranophosphate, phosphoramidite, condensation, boronation, terminal deprotection, Leishmania glycocalyx lipophosphoglycans | ||||||||
| There are multiple phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000315 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1021/jo102584g | |||||||
PubMed ID |
21381786 | |||||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (8): 2648-59. | |||||||
Article Title |
Synthesis of oligo(α-D-glycosyl phosphate) derivatives by a phosphoramidite method via boranophosphate intermediates. | |||||||
Author |
Shoichi, Fujita; Natsuhisa, Oka; Fumiko, Matsumura; Takeshi, Wada | |||||||
Affiliation |
Department of Medical Genome Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Bioscience Building 702, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan. | |||||||
Reference Id |
REF-0000-000316 | |||||||
Source |
J. Org. Chem. 2011, 76, 2648-2659 | |||||||
Doi |
10.1021/jo102584g | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|