
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002064 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002064

|
||||
Regist Date |
2012/06/21 18:30:38 | ||||
| REACTANT | |||||
|
|
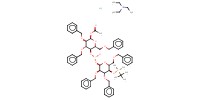
|
||||
Mol |
73 micro mol | ||||
|
|
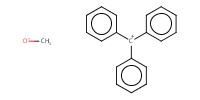
|
||||
Reactant Type |
TrOMe | ||||
Mol |
0.23 mmol | ||||
|
|
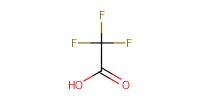
|
||||
Reactant Type |
TFA | ||||
Volume |
27 micro L | ||||
| PRODUCT | |||||
MOLECULE ID |
|
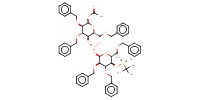
|
|||
Product Type |
intermediate | ||||
Yield |
85%(at least) | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour | ||||
Reaction Temp |
0 degree C | ||||
Solvent |
dry CH2Cl2 | ||||
| COMMENT | |||||
| Keywords: alpha-D-glycosyl boranophosphate, phosphoramidite, condensation, boronation, terminal deprotection, Leishmania glycocalyx lipophosphoglycans | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000315 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102584g | ||||
PubMed ID |
21381786 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (8): 2648-59. | ||||
Article Title |
Synthesis of oligo(α-D-glycosyl phosphate) derivatives by a phosphoramidite method via boranophosphate intermediates. | ||||
Author |
Shoichi, Fujita; Natsuhisa, Oka; Fumiko, Matsumura; Takeshi, Wada | ||||
Affiliation |
Department of Medical Genome Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Bioscience Building 702, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8562, Japan. | ||||
Reference Id |
REF-0000-000316 | ||||
Source |
J. Org. Chem. 2011, 76, 2648-2659 | ||||
Doi |
10.1021/jo102584g | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|