
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0002010 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-002010

|
||||
Regist Date |
2012/06/21 18:24:39 | ||||
| REACTANT | |||||
|
|
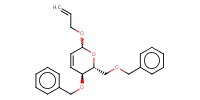
|
||||
Mol |
1 equiv. | ||||
|
|
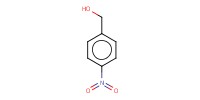
|
||||
Mol |
1.5 equiv. | ||||
|
|
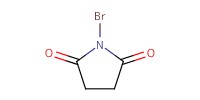
|
||||
Reactant Type |
NXS | ||||
Mol |
1.2 equiv. | ||||
|
|
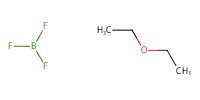
|
||||
Reactant Type |
catalyst | ||||
Mol |
10 mol % | ||||
| PRODUCT | |||||
MOLECULE ID |
|
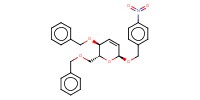
|
|||
Yield |
75% | ||||
| REACTION DETAIL | |||||
Reaction Time |
10 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
CH2Cl2 | ||||
| COMMENT | |||||
| Keywords: NBS, Lewis acid, 2,3-unsaturated allyl glycosides, stereoselective alpha-glycosylation, unsaturated sugars | |||||
| ATTENTION: There is a typo in the table. (BF3(OEt)2 should be BF3*OEt2) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000313 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo102333x | ||||
PubMed ID |
21438621 | ||||
Journal Name |
The Journal of organic chemistry. (2011) 76 (9): 3506-10. | ||||
Article Title |
2,3-Unsaturated allyl glycosides as glycosyl donors for selective α-glycosylation. | ||||
Author |
Brijesh, Kumar; Mushtaq A, Aga; Abdul, Rouf; Bhahwal A, Shah; Subhash C, Taneja | ||||
Affiliation |
Bio-organic Chemistry Division, Indian Institute of Integrative Medicine (CSIR), Canal Road, Jammu, India-180001. | ||||
Reference Id |
REF-0000-000314 | ||||
Source |
J. Org. Chem. 2011, 76, 3506-3510 | ||||
Doi |
10.1021/jo102333x | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|