
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001914 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001914

|
||||
Regist Date |
2012/06/21 18:14:21 | ||||
| REACTANT | |||||
|
|
|
||||
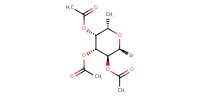
Reactant Type |
bromide | ||||
Mol |
9.54 mmol | ||||
|
|
|
||||
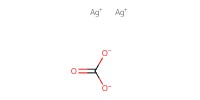
Reactant Type |
Ag2CO3 | ||||
Weight |
5 g | ||||
|
|
|
||||
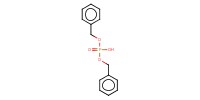
Reactant Type |
HO-P(O)(OBn)2 | ||||
Weight |
5 g | ||||
| PRODUCT | |||||
MOLECULE ID |
|
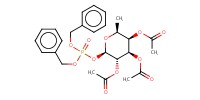
|
|||
Yield |
74% | ||||
| REACTION DETAIL | |||||
Reaction Time |
24 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
dry CH2Cl2/dry MeCN/dry diethyl ether = 150mL/150mL/15mL | ||||
Comment |
MS 3A was included in the solvent. | ||||
| The bromide and MS 3A were mixed and stirred for 30 minutes before the reaction. | |||||
| The reaction was conducted in dark condition. | |||||
| COMMENT | |||||
| Keywords: synthesis, Koenigs-Knorr method, silver carbonate | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000253 | ||||
Source |
Essentials of Carbohydrate Chemistry and Biochemistry | ||||
Reference Id |
REF-0000-000254 | ||||
Source |
ISBN 978-3-527-31528-4 | ||||
Reference Id |
REF-0000-000290 | ||||
Source |
Liebigs Ann. Chem. 121 (1991) | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|