
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001835 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001835

|
|||||||
Regist Date |
2012/06/21 18:07:19 | |||||||
| REACTANT | ||||||||
|
|
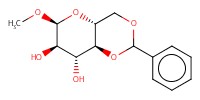
|
|
||||||
|
|
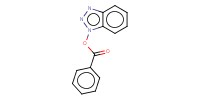
|
|||||||
|
|
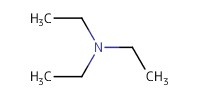
|
|||||||
Reactant Type |
Et3N | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
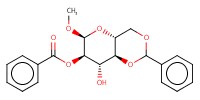
|
|
|||||
Yield |
95% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
NOT specified | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
CH2Cl2 | |||||||
Comment |
Very few were described regarding this reaction. | |||||||
| COMMENT | ||||||||
| Keywords: synthesis, regioselective acylation, hexopyranosides, triazole | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000253 | |||||||
Source |
Essentials of Carbohydrate Chemistry and Biochemistry | |||||||
Reference Id |
REF-0000-000254 | |||||||
Source |
ISBN 978-3-527-31528-4 | |||||||
Reference Id |
REF-0000-000262 | |||||||
Source |
Synthesis 1015 (1991) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|