
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001829 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001829

|
|||||||
Regist Date |
2012/06/21 18:06:52 | |||||||
| REACTANT | ||||||||
|
|
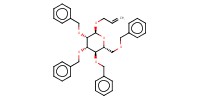
|
|
||||||
Reactant Type |
mannopyranoside | |||||||
Mol |
12.7 mmol | |||||||
|
|

|
|||||||
Reactant Type |
Pd on C (10%) | |||||||
Weight |
1.6 g | |||||||
|
|
|
|||||||
Reactant Type |
p-TsOH | |||||||
Mol |
5.96 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
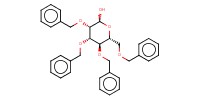
|
||||||
Yield |
65% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
3 hours | |||||||
Reaction Temp |
reflux | |||||||
Solvent |
85% aq. MeOH | |||||||
| COMMENT | ||||||||
| Keywords: synthesis, Fischer glycosylation, allyl glycosides, isomerization, 2,3,4,6-tetra-O-benzyl-alpha-D-mannopyranose | ||||||||
| The reaction is the 3rd part of the sequence. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000253 | |||||||
Source |
Essentials of Carbohydrate Chemistry and Biochemistry | |||||||
Reference Id |
REF-0000-000254 | |||||||
Source |
ISBN 978-3-527-31528-4 | |||||||
Reference Id |
REF-0000-000258 | |||||||
Source |
J. Carbohydr. Chem. 16, 877 (1997) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|