
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001820 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001820

|
||||
Regist Date |
2012/06/21 18:05:05 | ||||
| REACTANT | |||||
|
|
|
||||
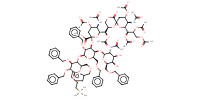
Mol |
0.25 mmol | ||||
|
|
|
||||
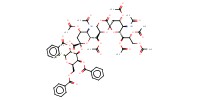
Mol |
0.38 mmol | ||||
|
|
|
||||
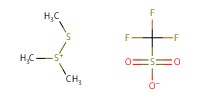
Reactant Type |
DMTST | ||||
Mol |
0.76 mmol | ||||
| PRODUCT | |||||
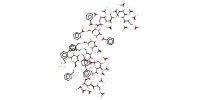
MOLECULE ID |
|
|
|||
Yield |
52% | ||||
| REACTION DETAIL | |||||
Reaction Time |
2 days | ||||
Reaction Temp |
0 degree C | ||||
Solvent |
CH2Cl2 | ||||
Comment |
53, 35, and MS 4A were mixed and stirred for 5 hours at room temperature before the reaction. | ||||
| MS 4A was included in the solvent. | |||||
| COMMENT | |||||
| Keywords: synthesis, sialyl glycosides, ganglioside GQ1b oligosaccharide | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000196 | ||||
Source |
PREPARATIVE CARBOHYDRATE CHEMISTRY | ||||
Reference Id |
REF-0000-000197 | ||||
Source |
ISBN 0-8247-9802-3 | ||||
Reference Id |
REF-0000-000250 | ||||
Source |
Tetrahedron Asymm. 5:2493 (1994) | ||||
Reference Id |
REF-0000-000251 | ||||
Source |
Carbohydr. Res. 260:C1 (1994) | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|